Electrochemistry
Define Galvanic cell /Daniell cell/Voltaic cell.
Cell that converts the chemical energy into electrical energy is called Galvanic cell /Daniell cell/Voltaic cell.
Chemical reactions in Daniell cell.
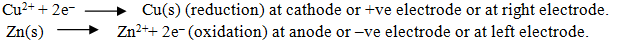
How can we represent the Galvanic cell.
Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s)
Define standard cell potential or emfo or Eo
The potential difference between the two electrodes of a galvanic cell is called the standard cell potential when concentration of electrolyte is unity. It is called the cell electromotive force(emf) of the cell when no current is drawn through the cell.
The standard cell potential is the difference between the electrode potentials (reduction potentials) of the cathode and anode.
Eocell = Eoright – Eoleft
Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s)
Eocell = Eoright – Eoleft = EoAg+|Ag – EoCu2+|Cu
Define standard hydrogen electrode(SHE).
Standard hydrogen electrode is a half-cell represented by Pt(s) H2(g) I H+(aq) has zero potential at all temperatures.
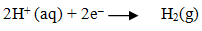
What do you mean by half cell.
Metal electrode dipped into salt solution which is present either in left side or right side of Galvanic cell is called half cell.
Example :In Cu(s)|Cu2+(aq)||Ag+(aq)|Ag(s)
Cu(s)|Cu2+(aq) is half cell present in right side.
Ag+(aq)|Ag(s) half cell present in left side.
How can we calculate standard half cell potential of half cell.
The standard potential of individual half-cell cannot be find. At 298 K the emf of the cell, standard hydrogen electrode is taken as anode and the other half-cell as cathode, gives the reduction potential of the other half-cell.
We can find EoCu2+ I Cu by following cell.
Pt(s)|H2(g) (1 bar) | H+(aq)1 M II Cu2+ (1M)|Cu .
Eocell = Eoright – Eoleft
EoCu2+|Cu(S) – EoH+|H2
= EoCu2+ |Cu(S)– 0
hence Eocell = EoCu2+|Cu(S)
How can we determine the cell potential (Ecell) of Galvanic cell by Nerns’t equation.

How we can determine the oxidizing and reducing power of substance according to their Eo value.
Higher the Eo(standard reduction potential) value higher the oxidizing power of that element.
Lower the Eo(standard reduction potential) value higher the reducing power of that element.
Free Energy and Cell Potential

The Relationship between Cell Potential & the Equilibrium Constant
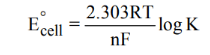
Define electrolytic cell .
The cell which converts electrical energy into chemical energy is called electrolytic cell.
Explain electrolytic cell with reaction involving at two electrodes.
We have a container containing an electrolyte a molten NaCl. In this electrolyte two platinum electrodes are dipped. One electrode which is joined to the positive terminal of battery is +ve electrode or anode and that joined to the negative terminal of battery is negative electrode or cathode.

Define conductance(G)
Reciprocal of resistance (R) is called conductance.
Unit of G=ohm -1 Or mho or ohm-1 .The SI unit of conductance is Siemens(s).
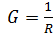
The SI unit of conductance is Siemens (S)
Define conductivity
The reciprocal of resistivity is called conductivity.Conductivity of a solution is defined as the conductance of a solution of 1 cm in length and area of cross-section 1 sq. cm
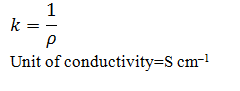
Define molar conductivity (ʌm)
The conductance of the electrolytic solution kept between the electrodes of a conductivity cell at unit distance but having area of cross section is large enough to contain volume of solution that contains one mole of the electrolyte.

Explain the relation between conductivity and concentration of electrolyte.
Conductivity increases with increase in concentration of both weak and strong electrolytes because the number of ions per unit in a solution increases on increasing concentration.
Explain the relation between molar conductivity and concentration of strong electrolyte.
ʌm increases slowly with dilution and can be represented by the equation.

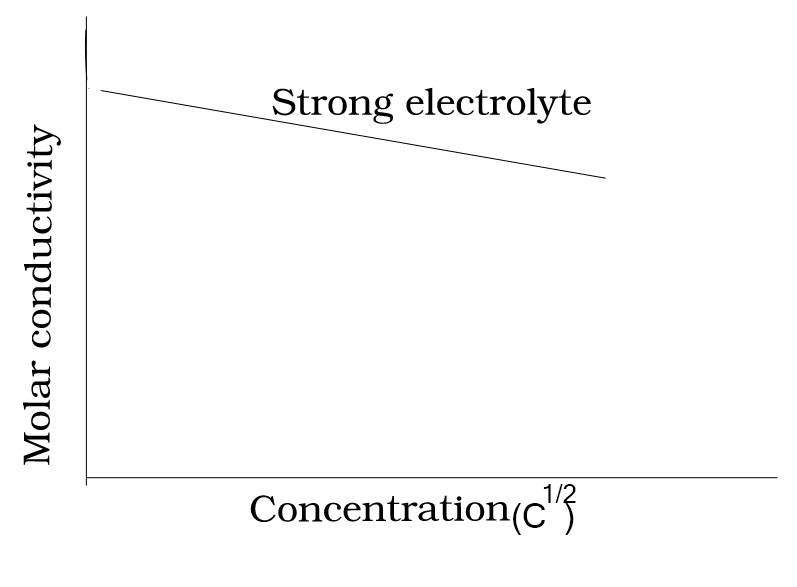
The value of the constant ‘A’ for a given solvent and temperature depends on the type of electrolyte. Molar conductivity increases with on dilution. It is due to the total volume, V, of solution containing one mole of electrolyte also increases.
Explain the relation between molar conductivity and concentration of weak electrolyte.

Molar conductivity increases with on dilution. It is due to the total volume, V, of solution containing one mole of electrolyte also increases. In such cases ʌm increases steeply on dilution, especially near lower concentrations. It is due to degree of dissociation of weak electrolyte depends on the concentration of electrolyte. As the concentration decreases degree of dissociation of electrolyte increases .
Define limiting molar conductivity
Molar conductivity of an electrolyte at infinite dilution is called limiting molar conductivity ![]()
Explain Kohlrausch law of independent migration of ions.
Limiting molar conductivity of an electrolyte is the sum of the individual contributions of the anion and cation of the electrolyte.

Explain application of Kohlrausch law of independent migration of ions.
Using Kohlrausch law of independent migration of ions, it is possible to calculate limiting molar conductivity of any electrolyte from the of individual ions.
How we can determine the value of degree of dissociation of weak electrolyte
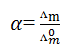
Define Faraday law of electrolysis.
Faraday’s irst Laws of Electrolysis
The amount of substance deposited on electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte .
w ∝ Q that is W=ZIt
Z=constant called electrochemical equivalent. I=current ,t=Time
Faraday’s Second Laws of Electrolysis
The amounts of different substances liberated by when same quantity of electricity is passed through the different electrolytic solution are proportional to their equivalent weights.

How much electricity is required to deposit one mole of substance at electrode.
nF electricity is required to deposit one mole of substance at electrode.
Where F =Faraday constant=96500 coulomb.
n=Number of electrons involved during the reaction.
Describe the factors effecting Product of electrolysis.
1. It depends on the nature of electrolyte.
2.The type of electrodes.
If the electrode is inert (e.g., platinum or gold), it does not participate in the chemical reaction and acts only as source for electrons. if the electrode is reactive, it participates in the electrode reaction.
3.Different oxidising and reducing species present in the electrolytic cell.
Define overpotential.
Ans.Some of the electrochemical processes although feasible, are so slow kinetically that at lower voltages these do not seem to take place and extra potential has to be applied, which makes such process more difficult to occur called overpotential.
What are the products of electrolysis if molten NaCl is taken.
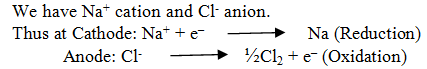
What are the products of electrolysis if aq NaCl is taken.
We have Na+ and H+cations and Cl– and OH–anions.
At Cathode: The reaction with higher value of Eo is preferred and therefore, the reaction at the cathode during electrolysis is:
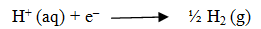
At anode :The reaction with lower value of Eo is preferred and therefore, OH– should oxidized but account overpotential the reaction at the cathode during electrolysis is:
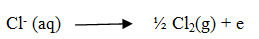
What are the products of electrolysis if aq sulphuric acid is taken.
We have H+cation and SO4 2– and OH–anions.
The reaction at the cathode during electrolysis is:
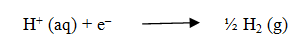
The reaction at the anode during electrolysis is when dilute sulphuric acid is taken
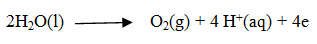
The reaction at the anode during electrolysis is when dilute sulphuric acid is taken :
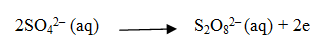
DefIne primary batteries.
The reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again. Examples dry cell or Leclanche cell.
DefIne secondary batteries.
A secondary cell after use can be recharged by passing current through it in the opposite direction so that it can be used again. Example The Lead storage battery.
Explain the structure and mechanism of dry cell.
Anode – zinc container
Cathode- carbon (graphite) rod surrounded by powdered manganese dioxide and carbon .
The space between the electrodes is filled by a moist paste of NH4Cl and ZnCl2

In the reaction at cathode, manganese is reduced from the + 4 oxidation state to the +3 state.
complex ion forms when ammonia produced in the dry cell reaction reacts Zn2+ is [Zn (NH3)4]2+.
Explain the structure and mechanism of Mercury cell.
Anode-zinc – mercury amalgam
Cathode-paste of HgO and carbon.
The electrolyte is a paste of KOH and ZnO.
The electrode reactions for the cell are given below:
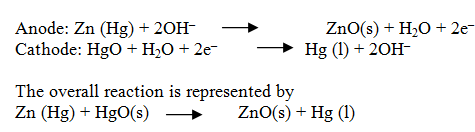
Explain the structure and mechanism of Lead storage battery when it is discharging.
Anode- lead
Cathode- grid of lead packed with lead dioxide (PbO2 )
A 38% solution of sulphuric acid is used as an electrolyte.
The cell reactions:
Explain the structure and mechanism of Lead storage battery when it is charging.

Define fuel cells and explain the structure and mechanism of this cell.
Galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy are called fuel cells.
In the cell, hydrogen and oxygen are bubbled through porous carbon electrodes into concentrated aqueous sodium hydroxide solution.
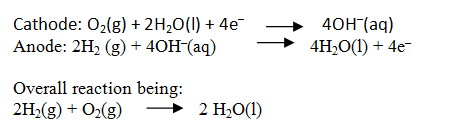
Explain the mechanism of corrosion.
Slowly eating away of the surfaces of metallic objects into oxides or other salts of the metal in presence of moisture is called corrosion.
Corrosion of Iron is called Rusting.
Mechanism:
At a particular spot of an object made of iron, oxidation takes place and that spot behaves as anode.

Electrons released at anodic spot move through the metal and go to another spot on the metal and reduce oxygen in presence of H+.
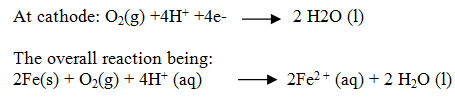
The ferrous ions are further oxidised by atmospheric oxygen to ferric ions which come out as rust in the form of hydrated ferric oxide (Fe2O3.xH2O)
Methods of prevention of corrosion .
1.To prevent the surface of the metallic object to come in contact with atmosphere. Cover it with paint or by some chemicals (e.g. bisphenol).
2. To cover the surface by other metals (Sn, Zn, etc.) that are inert or react to save the object.
3. Provide a sacrificial electrode of another metal (like Mg, Zn, etc.) which corrodes itself but saves the object.
