General principle and Processes of Isolation of Elements
Q.1.Define minerals.
Ans.Inorganic solid substance which are naturally occurring chemical substances in the earth’s crust obtainable by mining called minerals.
Q.2.Define ores.
Ans.A naturally occurring solid material from which a metal or valuable mineral can be profitably extracted.
Q.3.Define gangue.
Ans.Ores contaminated with earthly or undesired materials known as gangue.
Q.4.Define metallurgy.
Ans.The entire scientific and technological process used for isolation of the metal from its ores is known a metallurgy.
Q.5.Explain the different processes of metallurgy.
Ans.The extraction and isolation of metals from ores involve the following major steps:
- Concentration of the ore,
- Isolation of the metal from its concentrated ore, and
- Purification of the metal.
Q.6.Name the most abundant element of earth.
Ans.Aluminium
Q.7.Which ore is chosen for the extraction of aluminium.
Ans.Bauxite is chosen for aluminium.
Q.8.Which ore is chosen for the extraction of iron.
Ans.Iron,oxide ores are taken, which are abundant and do not produce polluting gases like SO2 that is produced in case iron pyrites.
Q.9.Define the term concentration/dressing/benefaction.
Ans. Removal of the unwanted materials e.g., sand, clays, etc,.from the ore is known as concentration, dressing or benefaction.
Q.10.What are the different types of concentration process.
Ans.
- Hydraulic washing
- Magnetic separation.
- Froth floatation method
- Leaching
Q.11.Explain the hydraulic process.
Ans.An upward stream of running water is used to wash the powdered ore. The lighter gangue particles are washed away and the heavier ores are left behind.
Q.12.Explain the Magnetic separation process.
Ans.The ground ore is carried on a conveyer belt which passes over a magnetic roller If either the ore or the gangue is capable of being attracted by a magnetic get separated by attracting towards magnetic field.
Q.13.Explain the froth floatation method.
Ans.This method is used for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water. Pine oils, fatty acids, xanthates or any other collector is added to this suspension.Cerosols,aniline or any other froth stabilizer is also added to it.Froth forms which contain minerals when this suspension is rotated by rotating paddle.The froth is light and thus skimmed off from suspension.
Q.14.Why is pine oils, fatty acids, xanthates or any other collector is added to this suspension in froth floatation method?
Ans.To enhance non-wettability of the mineral particles in water.
Q.15. How is ZnS separated from PbS when they are present on same ore by froth floatation method?
Ans NaCN depressant is used. It selectively prevents ZnS from coming to the froth but allows PbS to come with the froth.
Q.16.What is depressant which is used in froth floatation method in some cases.
Ans. It is possible to separate two sulphide ores by adjusting proportion of oil to water or by using depressants?
Q.17.What is leaching?
Ans. Leaching is the method used for concentrate the ore. If the ore is soluble in some suitable solvent this method is carried out.
Q.18.How is leaching of alumina from bauxite carried out?
Ans. Bauxite which contains SiO2,iron oxides and titanium oxide (TiO2) as impurities is added to concentrated NaOH solution. Al2O3 is leached out as sodium aluminate (and SiO2 too as sodium silicate) leaving the impurities behind.

The aluminate in solution is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated.
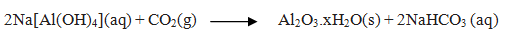
The sodium silicate remains in the solution and hydrated alumina is filtered, dried and heated to give back pure Al2O3.

Q.19. How is leaching of silver carried out?
Ans. Silver is leached with a dilute solution of NaCN or KCN in the presence of air from which the metal is obtained by replacement
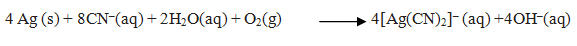
Q. 20.How does leaching of gold is carried out.
Ans Silver is leached with a dilute solution of NaCN in the presence of air from which the metal is obtained by replacement
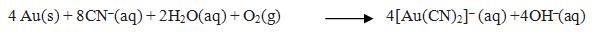
Q.21.Use of roasting and calcination.
Ans. While calcination is mostly used in the oxidation of carbonates, roasting is a method that is used for converting sulphide ores. During roasting, moisture and non-metallic impurities in the form of volatile gases are released.
Q.22.Define calcination.
Ans. Calcination is method of removal of a volatile fraction from concentrated ore and conversion into oxide.
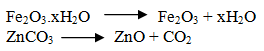
Q.23.Define Roasting.
Ans. The process of conversion of concentrated ore into oxide by heating the ore in regular supply of air in a furnace at a temperature below the melting point of the metal.
2ZnS + 3O2 → 2ZnO + 2SO2
2PbS + 3O2 → 2PbO + 2SO2
2Cu2S +3O2 → 2Cu2O + 2SO2
Q.24.What is copper matte?
Copper matte is a mixture of copper sulfide (Cu2S) and some iron sulfide (FeS). Matte is a process in which copper is extracted before the final reduction.
when a hot blast of air is blown through a molten matte placed in a silica lined converter, FeS of the matte oxidizes to FeO. This FeO combines with SiO2 (silica) to produce FeSiO3;(slag).
2FeS + 3O2 ⇒ 2FeO + 2SO2
FeO + SiO2 ⇒ FeSiO3
Q.25.How is metal obtained from its oxide.
Ans. (I) Direct heating of metal oxide into metal (Metals low in activity series)
2HgO → Hg +O2
(II) Using C or CO as reducing agent to reduce metal oxide into metal (Metals in the middle of the activity series)
MxOy + yC → xM + y CO
(III) Metal oxides are reduced to metal by electrolysis method(Metals in the top of the activity series)
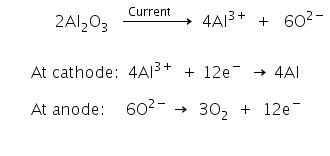
Q.26.Suggest a condition under which magnesium could reduce alumina.
Ans.The three equations are:
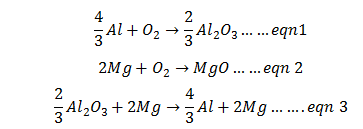
Reverse the eqn. number 1 and add it with eqn number 2 we get desired eqn. 3
From Ellingham diagram we can say that below1350oC, ΔG° value is –ve and reaction is feasible.
Q.27.Suggest a condition under which aluminum could reduce MgO.
Ans.The three equations are:
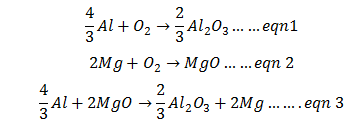
Reverse the eqn. number 2 and add it with eqn number 1 we get desired eqn. 3
From Ellingham diagram we can say that above 1350oC, ΔG° value is –ve and reaction is feasible.
Q.28.Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of aluminum why ?
Ans.Temperatures below the point of intersection of Al2O3 and MgO curves, magnesium can reduce alumina,but the process will be uneconomical.
Q.29.Why is the reduction of a metal oxide easier if the metal formed is in liquid state at the temperature of reduction?
Ans The entropy is higher if the metal is in liquid state than when it is in solid state. The value of entropy change (ΔS) of the reduction process is more on +ve side when the metal formed is in liquid state and the metal oxide being reduced is in solid state. Thus the value of ΔGo becomes more on negative side and the reduction becomes easier.
Q.30.Is following reaction feasible?
FeO(s) + C(s) → Fe(s) + CO (g)
Ans. Consider the two reactions
FeO(s) → Fe(s) +1/2O2(g)——-1
C(S)+1/2O2(g) → CO————2
Adding these two equation we get desired eqn.and value of ΔGo is –ve from Ellingham diagram thus reaction is feasible.
Q.31.How is Iron extracted from haematite.
Ans.The main iron ore is haematite. Haematite is iron(III) oxide Fe2O3.The iron ore contains impurities, mainly silica (silicon dioxide).Limestone (calcium carbonate) is added to the iron ore which reacts with the silica to form molten calcium
in the blast furnace.The calcium silicate (called slag) floats on the liquid iron.Since iron is below carbon in the reactivity series,iron in the ore is reduced to iron metal by heating with carbon (coke). It is actually carbon monoxide which does the reducing in the blast furnace. Hot air is blasted into the furnace causing coke (carbon) to burn rapidly producing carbon dioxide and raising the temperature to 2000oC.
The temperature where the reduction takes place is above 1500 °C. Iron falls to the bottom of the furnace where the temperature is approximately 2000 °C. Iron is liquid at this temperature and is tapped off periodically.
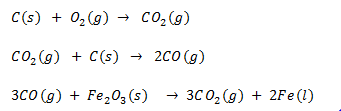
Q .32.Why is lime stone added tom blast furnace.
Ans Limestone is calcium carbonate (CaCO3) and it is added to the blast furnace to remove the impurities in the iron ore.
CaCO3(s) → CaO(s) + CO2(g)
CaO(s) + SiO2(s) → CaSiO3(l)
Q.33.What is difference among pig iron ,cast iron, wrought iron or malleable iron?
Ans.Pig iron – It contains about 4% carbon and contains many impurities in smaller amount e.g., S, P, Si, Mn. It can cast into variety of shapes.
Cast iron–It contains about 3% carbon and made by melting pig iron with scrap iron and coke using hot air blast. It is extremely hard and brittle.
Wrought iron or malleable iron- Itis the purest form of commercial iron and is prepared from cast iron by oxidising impurities in a reverberatory furnace lined with haematite.
Q.34.How is Cu extracted from copper(I)oxide?
Ans.The ore is heated in a reverberatory furnace after mixing with silica. In the furnace,iron oxide ‘slags of’ as iron silicate and copper is produced in the form of copper matte.This contains Cu2S and FeS. Copper matte is then charged into silica lined convertor. Some silica is also added and hot air blast is blown to convert the remaining Fes.FeO,and Cu2S/Cu2O to the metallic copper.
Following reaction takes place:
| 2FeS + 3O2 → 2FeO + 2SO2 |
| FeO + SiO2 → FeSiO3 |
| 2Cu2S + 3O2 → + 2SO2 |
| 2Cu2O + Cu2S → 6Cu + SO2 |
Q.35.How is zinc extracted from zinc oxide?
The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.

The metal is distilled off and collected by rapid chilling.
Q.36.In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AlF6 (Cryolite) or CaF2 why?
Ans .They lowers the melting point of the mix and brings conductivity.
Q.37.Describe Hall-Heroult process for the purification of Aluminum.
Ans.Cathode : Steel vessel with lining of carbon.
Anode: graphite
Electrolyte: Molten Al2O3 with Na3AlF6
Cathode: Al3+ (melt) + 3e– → Al(l)
Anode: C(s) + O2– (melt) → CO(g) + 2e–
C(s) + 2O2– (melt) → CO2 (g) + 4e–
Overall Process: 2Al2O3 + 3C → 4Al + 3CO2
Q.38.At a site, low grade copper ores are available and zinc and iron scraps are also available. Which of the two scraps would be more suitable for reducing the leached copper ore and why?
Ans Zinc being above iron in the electrochemical series (more reactive metal is zinc), the reduction will be faster in case zinc scraps are used. But zinc is costlier metal than iron so using iron scraps will be advisable and advantageous.
Q.39.Describe the extraction of chlorine from brine (chlorine is abundant in sea water as common salt)?
Ans.Aq solution of NaCl is eloctrolysed using graphite electrodes. But the electrolysis requires an excess potential to overcome some other hindering reactions. Thus, Cl2 is obtained by electrolysis giving out H2 and aqueous NaOH as by products.
2Cl–(aq) + 2H2O(l) → 2OH–(aq) + H2(g) + Cl2(g)
Q.40.What happenes electrolysis of molten NaCl is carried out.
Ans.Na metal is produced at cathode and not NaOH.
Q.41.Define Distillation
Ans.This is very useful for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
Q.42.Define Liquation.
In this method a low melting metal like tin, it can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
Q.43.Explain electrolyting refining.
Ans In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. They are put in a suitable electrolytic bath containing soluble salt of the same metal. The more basic metal remains in the solution and the less basic ones go to the anode mud.
Anode : M → Mn+ + ne–
Cathode: Mn+ + ne– → M
Q.44.How is copper refined using an electrolytic method.
Ans.Anodes are of impure copper and pure copper strips are taken as cathode. The electrolyte is acidified solution of copper sulphate and the net result of electrolysis is the transfer of copper in pure form from the anode to the cathode:
Anode: Cu → Cu2+ + 2 e–
Cathode: Cu2+ + 2e– → Cu
Q.45.Explain Zone refining.
Ans.This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. A circular mobile heater is fixed at one end of a rod of the impure metal.The molten zone moves along with the heater which is moved forward. As the heater moves forward, the pure metal crystallises out of the melt and the impurities pass on into the adjacent molten zone.
Q.46.Explain vapour phase refining.
Ans.In this method, the metal is converted into its volatile compound and collected elsewhere. It is then decomposed to give pure metal.
Q.47.What are the two requirements for vapour phase refining?
Ans.(i) The metal should form a volatile compound with an available reagent,
(ii) The volatile compound should be easily decomposable, so that the recovery is easy.
Q.48.Explain Mond Process for Refining Nickel.
Ans. In this process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetracarbonyl:
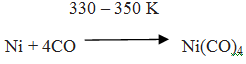
The carbonyl is subjected to higher temperature so that it is decomposed giving the pure metal:
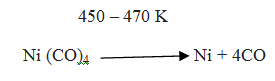
Q.49.Explain van arkel method for refining Zirconium or Titanium.
Ans. The crude metal is heated in an evacuated vessel with iodine. The metal iodide being more covalent, volatilises:
Zr + 2I2 → ZrI4
The metal iodide is decomposed on a tungsten filament, electrically heated to about 1800K. The pure metal is thus deposited on the filament.
ZrI4 → Zr + 2I2
Q .50.Explain chromatographic method.
Ans It is based on the principle that different components of a mixture are differently adsorbed on an adsorbent. The mixture is put in a liquid or gaseous medium which is moved through the adsorbent. Different components are adsorbed at different levels on the column. Later the adsorbed components are removed (eluted) by using suitable solvents (eluant).
Q.51.What is the advantage of column chromatography?
Ans.This is very useful for purification of the elements which are available in minute quantities and the impurities are not very different in chemical properties from the element to be purified.
Q.52.What are the uses of Aluminium,Copper, Zinc and Iron.
Ans.
Aluminium
- Aluminium foils are used as wrappers for chocolates.
- The fine dust of the metal is used in paints and lacquers.
- Aluminum, being highly reactive, is also used in the extraction of chromium and manganese from their oxides.
- Wires of aluminum are used as electricity conductors.
Copper
- Copper is used for making wires used in electrical industry and for water and steam pipes.
- It is also used in several alloys that are rather tougher than the metal itself
- e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
Zinc
- Zinc is used for galvanising iron.
- It is also used in large quantities in batteries
- As a constituent of many alloys
e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%).
- Zinc dust is used as a reducing agent in the manufacture of dye-stuffs
Cast iron
- It is the most important form of iron, is used for casting stoves, railway sleepers, gutter pipes , toys, etc.
- It is used in the manufacture of wrought iron and steel.
- Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements.
- Nickel steel is used for making cables, automobiles and aeroplane parts, pendulum, measuring tapes, chrome steel for cutting tools and machines.
- Stainless steel for cycles, automobiles, utensils pens etc.
