Redox Reactions
Define oxidation and reduction.
Definitions of Oxidation
Definition no 1: Addition of oxygen to an element or a compound called oxidation.

Definition:2 The removal of hydrogen from a substance called oxidation.
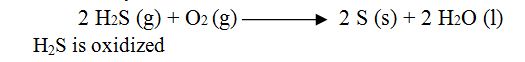
Definition 3 : Removal of electropositive element from substance is called oxidation.
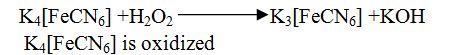
Definition 4: Addition of electronegative element to a substance is called oxidation.
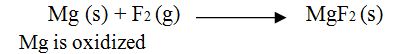
Definitions of Reduction.
Definition no1: Addition of hydrogen to an element or a compound is called reduction
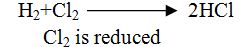
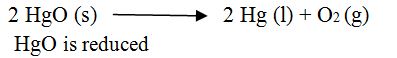
Definition:2 The removal of oxygen from a substance is called reduction
Definition 3: Addition of electropositive element from substance is called reduction.

Definition 4: Removal of electronegative element from substance is called reduction.

Define redox reactions.
When oxidation and reduction occur simultaneously that reaction is called redox reaction.
Define oxidation and reduction reactions in terms of electron transfer.
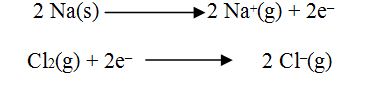
loss of electrons called oxidation and the gain of electrons is called reduction.
Define oxidizing agent and reducing agent.
Compound which oxidises other and reduces itself is called oxidizing agent.
Compound which reduces other and oxidises itself is called reducing agent.
Define Oxidation number.
Oxidation state of an element in a compound assuming there is a complete transfer of electron from a less electronegative atom to a more electonegative atom and determined by a set of rules.
Define oxidation,reduction,oxidizing agent ,reducing agent and redox reactions in terms of oxidation number.
Oxidation: Increase in oxidation number of the element in the given substance.
Reduction: Decrease in oxidation number of the element in the given substance.
Oxidising agent: A reagent which increases the oxidation number of an element in a given substance and decreases itself.
Reducing agent: A reagent which lowers the oxidation number of an element in a given substance and increases itself.
Redox reactions: Reactions in which there is increase in oxidation number of an element and also decrease in oxidation number takes place is called redox reduction.
What are the rules for calculation of oxidation number?
1. The oxidation number of an atom in free elements is zero. Hydrogen in H2, sulphur in S8, phosphorus in P4 oxygen in O3 is zero.
2.Fluorine has oxidation number -1
3. Oxidation number of oxygen is -2 in all compounds except in peroxides, superoxides and oxygen fluorides. Oxidation number in peroxide is -1, in superoxides -1/2, in OF2 +2 and in O2F2 is +1.
4.The oxidation number of hydrogen is +1 in all of its compounds except metallic hydrides. In metallic hydrides it is +1.
5.The oxidation number of an ion is equal to the charge on it.
6.The oxidation number of group 1 elements(Li,Na,K,Rb,Cs) is +1 and the oxidation number of group 2 elements(Be,Mg,Ca,Sr,Ba) is +2.
7.For complex ions, the algebraic sum of oxidation numbers of all the atoms is equal to the net charge on the ion.
8.For neutral molecules the algebraic sum of oxidation numbers of all the atoms is equal o zero.
Balance the following equation by oxidation number method in acidic medium.
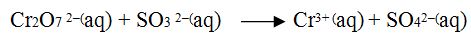
Step1.Calculate the oxidation number of each elements taking part in reaction.
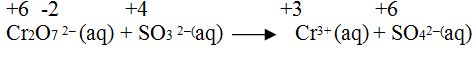
Step 2. Balance the atoms which oxidation number changed during the reaction.

Step.3 Calculate the increase or decrease in the oxidation number per atom which oxidation number changed during the reaction.
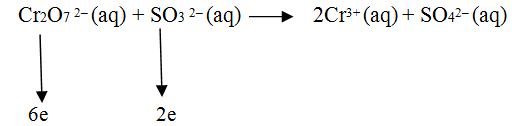
Step.4 If these changes are not equal then multiply by suitable number so that these become equal.
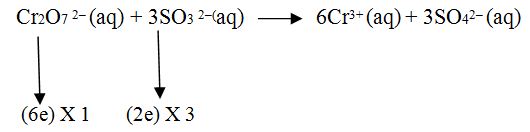
Step.5 Balance oxygen if unbalance.

Balance the following equation by ion electron method method/half reaction method in acidic medium.

Step1.Calculate the oxidation number of each elements taking part in reaction.
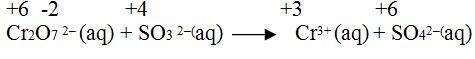
Step 2. Write individual oxidation and reduction reaction.

Step 3 : Balance the atoms which oxidation number changed during the reaction.

Step 4: Calculate the increase or decrease in the oxidation number per atom which oxidation number changed during the reaction and add it in higher oxidation state side.
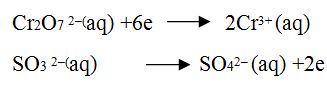
Step5. Add two reactions so that added electron must be cancelled.

Step6: Balance Oxygen atoms

Rule for balancing oxygen in acidic medium
Add H+ twice of the unbalance oxygen in that side in which higher number of oxygen is present and H2O equal number of unbalance oxygen in another side.
Rule for balancing oxygen in basic medium
Add H+ equal number of the unbalance oxygen in that side in which lower number of hydrogen is present.
Rule for balancing hydrogen in basic medium
Add OH– equal number of the unbalance oxygen in that side in which lower number of oxygen is present and H2O equal number of unbalance hydrogen in another side.
Rule for balancing hydrogen in basic medium
Add OH– equal number of the unbalance oxygen in that side in which higher number of oxygen is present and H2O equal number of unbalance hydrogen in another side.
Define electrolytic cell.
The cell which convert electrical energy into chemical energy is called electrolytic cell.
Define Galvanic cell.
The cell which convert chemical energy into electrical energy is called electrolytic cell.
Define electrode potential.
Potential of individual electrode is called electrode potential.
Dehine Standard Electrode Potential.
At 298K when the concentration of each species taking part in the electrode reaction is unity reaction is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential .
