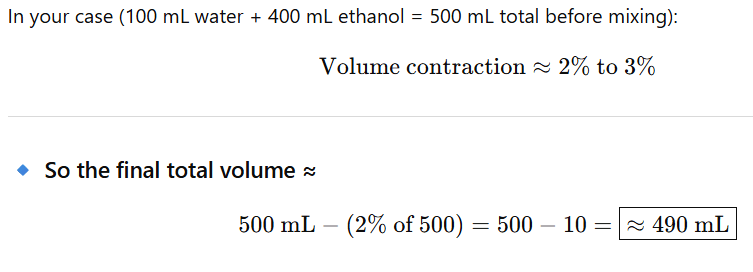
When 100 mL of water is mixed with 400 mL of ethanol, the total volume is not simply additive — because hydrogen bonding causes volume contraction.
Reason:
Water and ethanol molecules form strong hydrogen bonds, allowing the molecules to pack more closely.
So the mixture’s final volume is less than 500 mL.
Experimental result:
When equal amounts of water and ethanol are mixed, the volume contraction is about 4–5%.