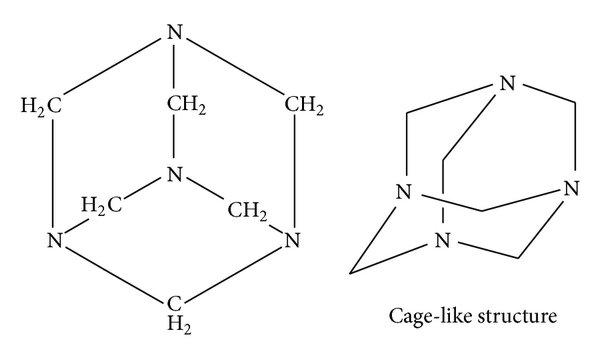
Urotropin is another name for hexamethylenetetramine (chemical formula: C₆H₁₂N₄).
🔹 Chemical Information
- IUPAC Name: Hexamethylenetetramine
- Molecular Formula: C₆H₁₂N₄
- Molar Mass: 140.19 g/mol
- Structure: It is a cage-like compound made up of four nitrogen atoms linked by methylene (-CH₂-) groups.
🔹 Preparation
Urotropin is formed by the reaction of formaldehyde with ammonia.
6HCHO + 4NH3→ C6H12N4 + 6H2O
🔹 Uses
- Medicine: Used in the treatment of urinary tract infections (UTIs) because in acidic urine, it releases formaldehyde, which acts as an antiseptic.
- Explosives: Used in the preparation of RDX (Cyclonite), a powerful explosive.
- Fuel tablets: Used in solid fuel tablets for camping and military use (burns smokelessly).
- Plastics & resins: Used in making phenol-formaldehyde resins.