Fructose is a reducing sugar, even though it’s a ketose (contains a keto group), not an aldehyde.
Let’s see how?
1. Reducing sugar meaning
A reducing sugar is one that can reduce mild oxidizing agents like Tollens’ reagent (Ag⁺ → Ag) or Fehling’s solution (Cu²⁺ → Cu⁺) — because it has a free aldehyde or ketone group (or can form one in solution).
2. Fructose has a ketone group (not aldehyde)
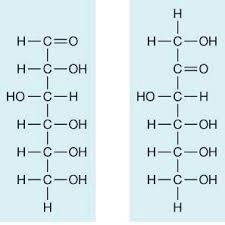
At first glance, this looks non-reducing because it lacks a free aldehyde group.
3. In alkaline medium → tautomerization
In basic solution, fructose undergoes keto–enol tautomerism, forming an enediol intermediate.
This intermediate can rearrange to give glucose and mannose, both of which are aldehydes (aldoses). Fructose⇌enediol formalkaline mediumGlucose+Mannose\text{Fructose} \xrightleftharpoons[\text{enediol form}]{\text{alkaline medium}} \text{Glucose} + \text{Mannose}Fructosealkaline mediumenediol formGlucose+Mannose
4. Hence → Reducing property
Because glucose and mannose can reduce Tollens’ and Fehling’s reagents, fructose also gives a positive test indirectly.
So, fructose is a reducing sugar — not directly, but via tautomeric conversion to an aldehyde sugar.
