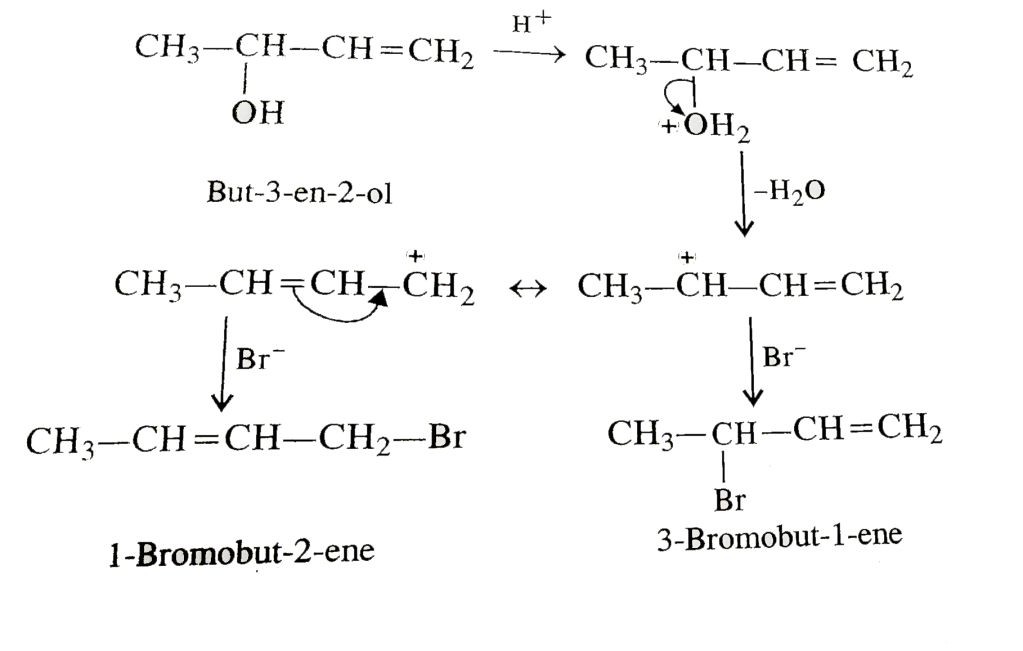
Major product: bromine replaces the –OH to give CH₃–CH(Br)–CH=CH₂ (IUPAC: 3-bromobut-1-ene) — with a minor product CH₃–CH=CH–CH₂Br (1-bromo-2-butene) possible.
Why (mechanism, stepwise):
- Protonation of the alcohol.
CH₃–CH(OH)–CH=CH₂ + H⁺ → CH₃–CH(OH₂⁺)–CH=CH₂ - Loss of water → allylic carbocation (RDS).
CH₃–CH(OH₂⁺)–CH=CH₂ → CH₃–C⁺H–CH=CH₂ (carbocation at C-2).
This carbocation is allylic and is resonance stabilized: the + charge is delocalized between C-2 and the terminal C-4 (move the π electrons C3–C4 → C2–C3). Resonance forms:- A: CH₃–C⁺H–CH=CH₂ (positive at C2)
- B: CH₃–CH=CH–CH₂⁺ (positive at terminal C4)
- Nucleophilic attack by Br⁻.
Bromide attacks the resonance-stabilized cation. Attack at C-2 (the more substituted resonance form) is favored, giving CH₃–CH(Br)–CH=CH₂ (major). Attack at the terminal C (resonance form B) gives CH₃–CH=CH–CH₂Br (minor).
Minor product is more stable alkene?
minor product can be the thermodynamically more stable alkene, but the reaction gives the major product for kinetic/reaction-path reasons. Here’s why, step-by-step, in plain language.
1) What the two products are (reminder)
- Major product (fast): bromide ends up on the more substituted carbon (attack at C-2) → gives CH₃–CH(Br)–CH=CH₂ (formed quickly by attack on the resonance form with the positive charge at C-2).
- Minor product (more stable alkene): bromide ends up at the terminal site (attack at C-4 resonance form) → gives CH₃–CH=CH–CH₂Br, which may correspond to the more substituted/thermodynamically more stable alkene after any isomerization.
2) Why the less stable alkene is the major product
- After loss of water you form an allylic carbocation that is resonance-stabilized. The two resonance contributors are not equally important: the contributor with the positive charge on C-2 (the more substituted position) is a major contributor (it has greater carbocation stabilization by adjacent alkyl groups). That means Br⁻ encounters more positive character at C-2, so nucleophilic attack there is faster. The product from that fast attack is the observed major product — this is kinetic control.
- The route that would give the thermodynamically more stable alkene requires attack on the resonance contributor with the charge at the terminal carbon (less favored contributor), so that pathway is slower → hence minor product.
3) Stability vs. kinetics
- Thermodynamic stability (more substituted internal alkene) and kinetic accessibility (where Br⁻ attacks fastest) are two different things. The reaction pathway here is under kinetic control (fast capture of the carbocation by Br⁻), so the kinetically favored product predominates even if it’s not the most stable alkene.
