When you first look at alcohols (ROH) and thiols (RSH), they seem quite similar — both contain a hydrogen attached to a group 16 element (oxygen or sulfur).
Yet, when it comes to acidity, thiols clearly win.
In this post, we’ll explore why thiols (RSH) are stronger acids than alcohols (ROH) — step by step.
Step 1: Acid strength depends on conjugate base stability
The strength of an acid depends on how stable its conjugate base is after losing a proton (H⁺).
- Alcohols (ROH) lose H⁺ to form alkoxide ions (RO⁻)
- Thiols (RSH) lose H⁺ to form thiolate ions (RS⁻)
The more stable the conjugate base, the stronger the acid.
So, we must compare the stability of RO⁻ and RS⁻.
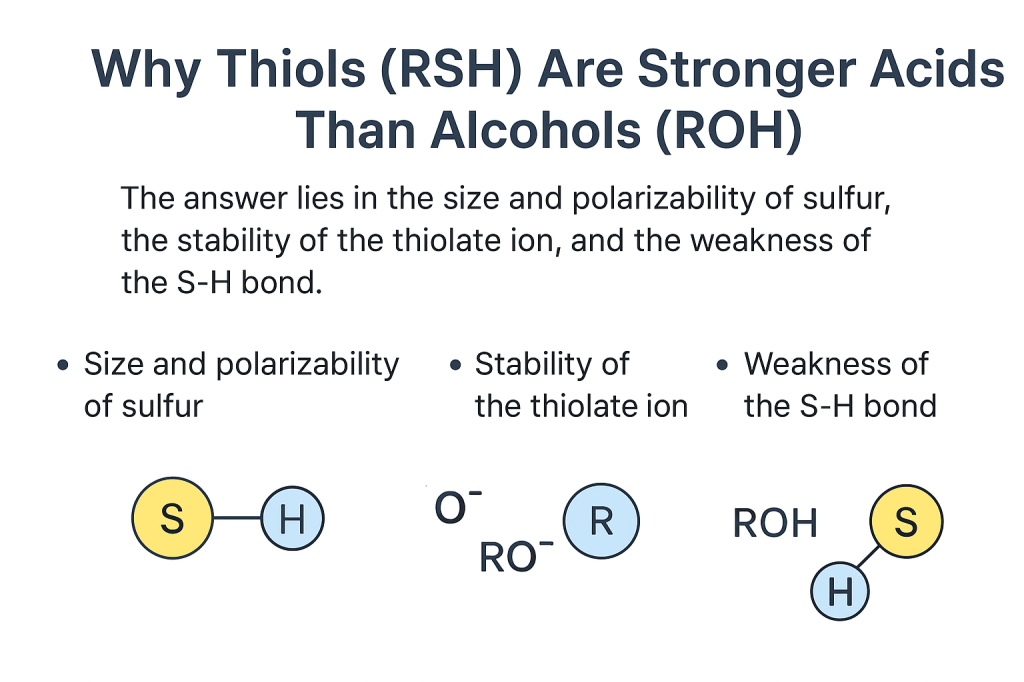
Step 2: The role of atom size and polarizability
Sulfur (S) is larger and more polarizable than oxygen (O).
That means the negative charge on sulfur in RS⁻ is spread over a larger volume, making it more stable.
In contrast, oxygen is smaller and holds the negative charge tightly on a small area, creating stronger charge density and less stability.
Result: RS⁻ is more stable than RO⁻ → RSH is a stronger acid.
Step 3: Bond strength difference
The S–H bond is weaker than the O–H bond.
It takes less energy to break the S–H bond and release a proton.
Weaker bond → easier H⁺ release → stronger acid
Step 4: Electronegativity vs Polarizability
Although oxygen is more electronegative than sulfur, electronegativity isn’t the main factor here.
For acid strength, charge delocalization and bond strength dominate — and sulfur’s large, polarizable nature stabilizes the anion far better than oxygen.
tep 5: pKa values tell the story
| Compound | Approx. pKa | Acid Strength |
|---|---|---|
| Methanol (CH₃OH) | ~16 | Weaker acid |
| Methanethiol (CH₃SH) | ~10 | Stronger acid |
A lower pKa means a stronger acid — so thiols are clearly more acidic than alcohols.
✅ Final Summary
| Factor | Alcohol (ROH) | Thiol (RSH) | Stronger Acid? |
|---|---|---|---|
| Atom size | Small (O) | Large (S) | ✅ RSH |
| Charge delocalization | Less | More | ✅ RSH |
| Bond strength | Strong O–H | Weak S–H | ✅ RSH |
| pKa | ~16 | ~10 | ✅ RSH |
Conclusion
Thiols (RSH) are stronger acids than alcohols (ROH) because their conjugate bases (RS⁻) are more stable.
Sulfur’s larger size and greater polarizability allow the negative charge to spread out, and the weaker S–H bond makes proton loss easier.
So, while oxygen wins in electronegativity, sulfur wins in stability, making RSH the stronger acid overall.
