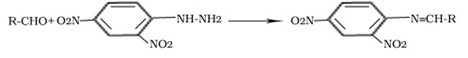
Reasoning based questions
Q.1.The boiling point of aldehydes and ketones are higher than hydrocarbons having comparable molecular masses why?
Ans. Boiling point of aldehydes and ketones are higher than that of hydrocarbons because they are polar and hydrocarbons are non-polar therefore, the intermolecular dipole-dipole attraction in aldehydes and ketones is stronger than hydrocarbons.
Q.2.The boiling point of aldehydes and ketones are higher than ethers having comparable molecular masses why?
Ans. Boiling point of aldehydes and ketones are higher than that of ethers because they are more polar than ethers therefore, the intermolecular dipole-dipole attraction in aldehydes and ketones is stronger than ethers.
Q.3. Boiling point of aldehydes and ketones are lower than those of alcohols of similar molecular masses why?
Ans. It is due to absence of intermolecular hydrogen bonding in aldehydes and ketones.
Q.4. The lower members of aldehydes and ketones such as methanal, ethanal and propanone are miscible in water why ?
Ans. Lower members of aldehydes and ketones are able to form hydrogen bond with water. As molecular mass increases hydrophobic alkyl part also increases thus solubility decreases.
Q.5. Arrange the following compounds in increasing order of their boiling point.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3
Ans . CH3CH2OH (Alcohol) > CH3CHO (Aldehyde) > CH3OCH3 (Ethers) > CH3CH2CH3 (Alkane)
Above order is due to alcohols have hydrogen bonding between their molecules. Aldehydes have dipole- dipole interaction and hydrocarbons are non polar.
Q.6. Aldehydes and ketones generally give nucleophilic addition reactions why?
Ans .It is due to electrophilic nature of carbon of carbonyl group.
Q.7. Aldehydes are generally more reactive than ketones in nucleophilic addition reactions why?
Ans. It is due to two reasons.
1.Steric hindrance effect of alkyl groups present at electrophilic carbon.
The presence of two relatively large substituents in ketones hinders the approach of nucleophile to carbonyl carbon.
2. +I effect of alkyl groups present at electrophilic carbon.
Two alkyl groups reduce the electrophilicity of the carbonyl carbon more effectively in ketones.
Q.8. Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
(i) Ethanal, Propanal, Propanone, Butanone.
(ii) Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Ans (i). Butanone < Propanone < Propanal < Ethanal.
Ethanal and Propanal are aldehyde hence they are more reactive than propanone and butanone. Electrophilic carbon of butanone has larger alkyl groups than propanone hence former is less reactive.
(ii) Acetophenone < p-Tolualdehyde < Benzaldehyde < p-Nitrobenzaldehyde
Above order is due to acetophenone is ketone. Pushing group (CH3) decreases the electrophilic nature of carbon of carbonyl group and that of pulling group (like NO2) increases.
Q.9. Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols having comparable molecular masses why ?
Ans. It is due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
Q.10. Simple aliphatic carboxylic acids having upto four carbon atoms are miscible in water why?
Ans. It is due to the formation of hydrogen bonds with water. The solubility decreases with increasing number of carbon atoms. Higher carboxylic acids are insoluble in water due to the increased hydrophobic interaction of hydrocarbon part.
Q.11. Give the reactions which suggest carboxylic acids are acidic in nature.
Ans. CH3-COOH + Na → CH3COONa + H2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Q.12.What is Ka and PKa?
Ans. Ka is ionization constant of acid. Higher the value of Ka higher the acidic strength. PKa is –log Ka thus lower the PKa value higher the acidic strength.
Q.13.Carboxylic acids are more acidic than Phenol why?
Ans.

The carboxylate ion is more stabilized than phenoxide ion, so carboxylic acids are more acidic than phenols. The conjugate base of carboxylic acid, a carboxylate ion, is stabilised by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom. The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
Q.14.How does electron withdrawing group and electron releasing group affect the acidic strength of carboxylic acid?
Ans. Electron withdrawing groups increases the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects. Conversely, electron donating groups decrease the acidity by destabilising the conjugate base.
The effect of the following groups in increasing acidity order is
Ph < I < Br < Cl < F < CN < NO2 < CF3
Higher the carbon atoms in alkyl group higher the pushing effect.Thus, the following acids are arranged in order of increasing acidity
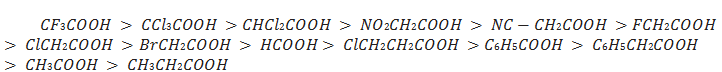
Q.15. Arrange the para-methoxybenzoic acid ,Benzoic acid and p-nitrobenzoic acid in decreasing order of acidic strength.
Ans.
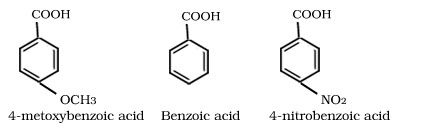
p-nitrobenzoic acid > benzoic acid > p-metoxybenzoic acid
Electron withdrawing group increases while electron donating groups decrease the acidic strength of benzoic acid.
Q.16.Which is more acidic in prop-2-enoic acid and propanoic acid?
Ans. Conjugate –ve ion of prop-2-enoic acid is extra resonance stabilized hence it is more acidic than propanoic acid.
Q.17.Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group why?
Ans.

Carboxyl group withdraw electrons from benzene ring by –R effect hence it is deactivating group since electron density is high at meta position hence it is meta directing.
Q.18.Benzoic acid do not undergo Friedel-Crafts reaction why?
Ans. Because the carboxyl group is deactivating and the catalyst aluminum chloride (Lewis acid) gets bonded to the carboxyl group).
Name Reactions in Aldehydes, Ketones and Carboxylic Acids
Rosenmund reduction
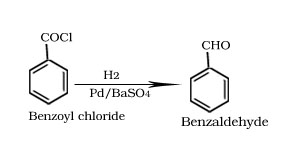
Stephen reaction
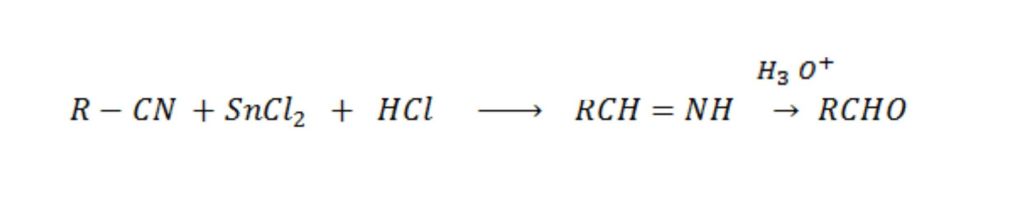
Etard reaction
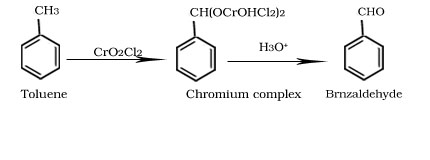
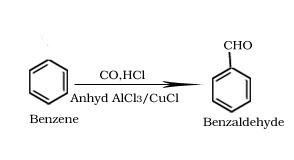
Gatterman-Koch reaction
Clemmensen reduction
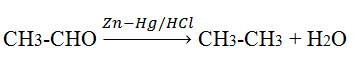
Wolff-Kishner reduction

Aldol condensation
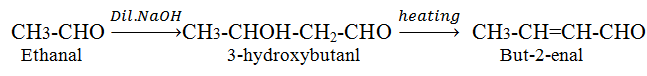
Cross aldol condensation
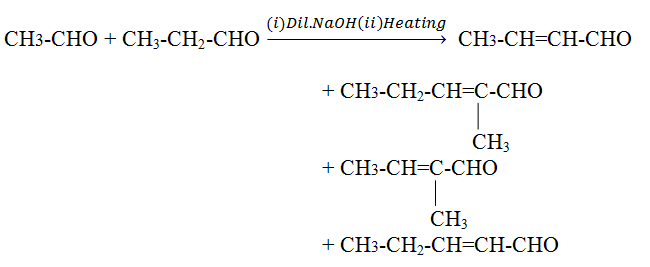
Cross aldol condensation
Cannizzaro reaction

Decarboxylation reaction

Hell-Volhard-Zelinsky reaction
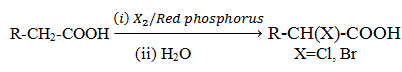
Distinguish reaction
Tollen’s reagent test
Distinguish between aldehydes and ketones.
Aldehydes react with Tollen’s reagent and give silver mirror test. Ketones give no reaction with Tollen’s reagent
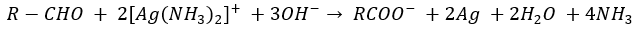
Fehling solution test
Distinguish between aldehydes and ketones.
Aldehydes react with Fehling reagent and give red brown ppt of Cu2O. Ketones give no reaction with Fehling reagent. Aromatic Aldehydes don’t give Fehling’s test.

Iodoform test
Distinguish between aldehydes and aldehydes and ‘ketones and ketones’.
Those aldehydes and ketones having CH3CO-give Iodoform test thus in aldehyde family only ethanal gives this test. In ketone family all methyl ketones give this test.
R-CO-CH3+ 3I2 + 4 NaOH → R-COONa +CHI3
Sodium hydrogencarbonate test
Distinguish between Carboxylic acid/Benzoic acid and alcohols/phenols
Carboxylic acids and benzoic acid react with sodium hydrogencarbonate give this test and release CO2. Alcohols and phenols don’t react with sodiumhydrogencarbonate.
R-COOH + NaHCO3 → R-COONa + H2O + CO2
Other reactions
Reduction of ester
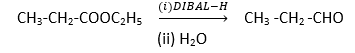
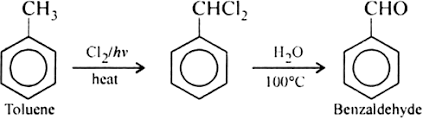
Formation of benzaldehyde from toluene

Formation of ketones from Grignard reagent.
2R-MgX + CdCl2→ R2Cd + 2Mg(X)Cl
R’-CO-Cl + R2Cd → R’-CO-R + CdCl2
CH3-CH2-CN + C2H5MgBr → CH3-CH2-C(C2H5)=NMgBr → CH3– CH2-CO-C2H5
Formation of ketone from benzene.
Nitration of benzaldehyde
Oxidation of Toluene
Hydrolysis of acid derivatives
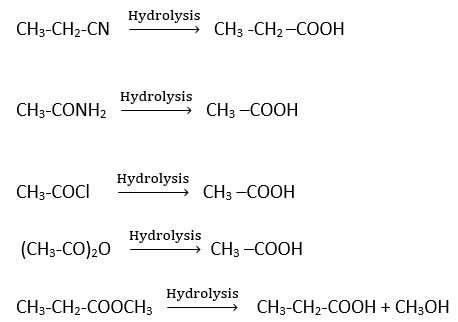
Formation of carboxylic acid from Grignard reagent.
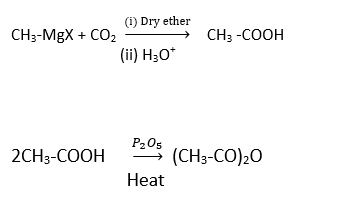
Formation of phthalimide
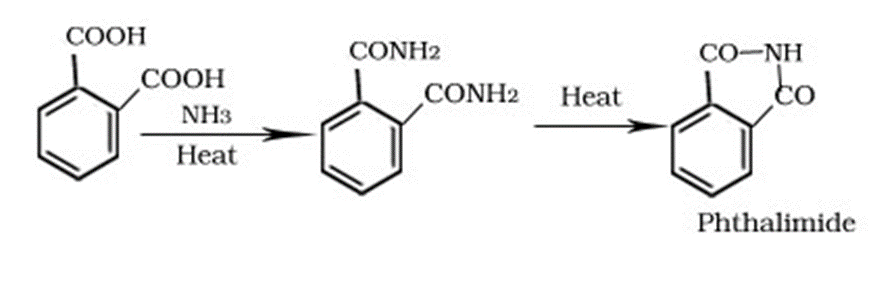
Reduction of Carboxylic Acids
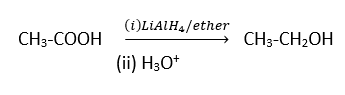
Nitration of Benzoic Acids
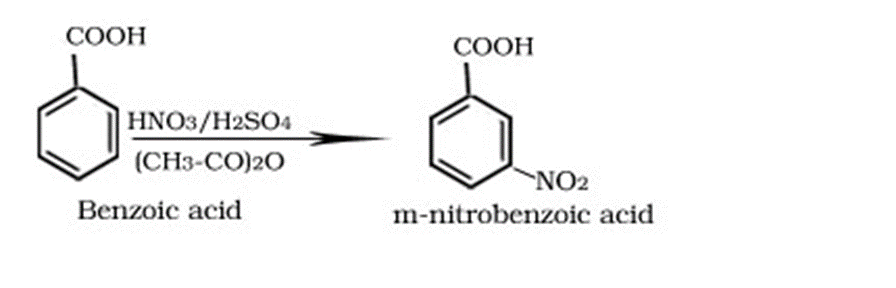
Halogenation of Benzoic Acids
Nucleophilic addition reaction of aldehydes and ketones
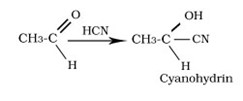
Reaction with HCN
Reaction with NaHSO3

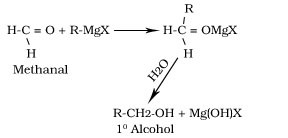
Reaction with Grignard reagent
Reaction with alcohol

Reaction with ammonia
R-CHO + NH3 → R-CH=NH
imine
Reaction with amine
R-CHO +R-NH2 → R-CH=N-R
Substituted imine (Schiff ‘Base)
Reaction with Hydroxyl amine
R-CHO + NH2OH → R-CH=N-OH
Oxime
Reaction with Hydarazine
R-CHO + NH2-NH2 → R-CH=N-NH2
Hydrazone
Reaction with semicarbazide
R-CHO +NH2-CO-NH-NH2 → R-CH=N -NH- CO-NH2
Senicarbazone
Reaction with Phenyl hydrazine
R-CHO +C6H5-NH-NH2 → R-CH=N -NH- C6H5
Phenyl hydrazone
Reaction with DNP 2,4-dinitrophenylhydrazine