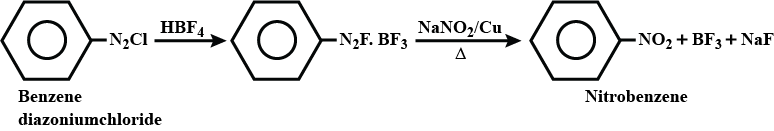Reasoning types question
Q.1.Ammonolysis method is not suitable for preparation of primary amines why?
Ans . Ammonolysis gives a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt hence this method is not suitable for preparation of primary amines.
Q.2. Arrange the order of reactivity of halides with amines RI, RBr, RCl.
Ans.The order of reactivity of halides with amines is RI > RBr > RCl.
Q.3. Aromatic primary amines cannot be prepared by Gabriel phthalimide synthesis.
Ans. Since C-X bond of aryl halides has partial double bond character due to resonance hence they don’t undergo nucleophilic substitution with the anion formed by phthalimide.
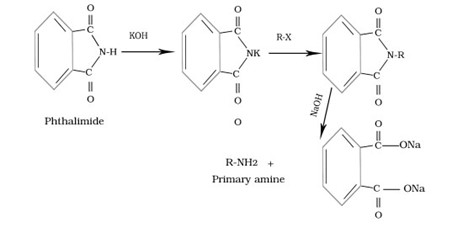
Q.4. Gabriel phthalimide synthesis is used for the preparation of primary amines why?
Ans.This method gives primary amine without any contamination of secondary and tertiary amine.
Q.5. Lower aliphatic amines are soluble in water why?
Ans. Because they can form hydrogen bonds with water molecules. However, solubility decreases with increase in molar mass of amines due to increase in size of the hydrophobic alkyl part.
Q.6.Which is more soluble in alcohol and amine if they have comparable molecular mass?
Ans. Since electronegativity of oxygen is higher than nitrogen hence alcohols are more polar than amines and form stronger intermolecular hydrogen bonds than amines hence alcohols are more soluble than amines.
Q.7.The order of boiling point of isomeric amines is as follows Primary > Secondary > Tertiary why?
Ans. The intermolecular association is more in primary amines than in secondary amines as there are two hydrogen atoms available for hydrogen bond formation in it. Tertiary amines do not have intermolecular association due to the absence of hydrogen atom available for hydrogen bond formation.
Q.8. Amines are basic in nature why?
Ans. It is due to the presence of a lone pair on nitrogen, it can react with acid and forms salt.
Q.9 .Compare basic strength between aliphatic amines and ammonia.
Ans. Due to electron releasing nature of alkyl group, it (R) pushes electrons towards nitrogen and thus makes the unshared electron pair more available for sharing with the proton of the acid thus aliphatic amines are more basic than ammonia.
Q.10.The order of basicity of amines in the gaseous phase follows the expected order
tertiary amine > secondary amine > primary amine > NH3.why?
Ans. Due to the electron releasing nature of alkyl group, it (R) pushes electrons towards nitrogen and thus makes the unshared electron pair more available for sharing with the proton of the acid. Since number of alkyl groups increases from primary amine to tertiary amine hence basic strength also increases in same order.
Q.11.The order of the basic strength in case of ethyl substituted amines in aqueous solution is as follows why?
(C2H5)2NH > (C2H5)3NH > (C2H5)3NH >NH3
Ans.The basic strength of amines depends on three factors.
- Inductive effect
- Solvation effect
- Steric hinderance of the alkyl group
Since steric hindrance effect of ethyl groups in tertiary amine make the stability of substituted ammonium cation lesser than that of secondary amine hence basic strength of tertiary amine is lower than secondary amine.
Q.12.The order of the basic strength in case of methyl substituted amines in aqueous solution is as follows why?
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
Ans.The basic strength of amines depends on three factors.
- Inductive effect
- solvation effect
- Steric hinderance of the alkyl group
When the alkyl group is small, like –CH3 group, there is no steric hindrance to H-bonding hence the stability of substituted ammonium cation in case of tertiary amine is lesser than that of primary amine hence basic strength of tertiary amine is lower than the primary amine.
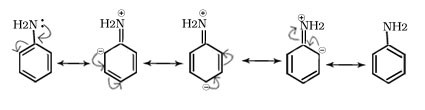
Q.13. Why is ammonia more basic than arylamines.
Ans. the -NH2 group is attached directly to the benzene ring. It results in the unshared electron pair on nitrogen atom to be in conjugation with the benzene ring and thus making it less available for protonation.
Q.14.What is the effect of electron releasing group and electron withdrawing group on basic strength of substituted aniline?
Ans. In case of substituted aniline, electron releasing groups like –OCH3, –CH3 increase the basic strength whereas electron withdrawing groups like –NO2, –SO3H, –COOH, –X decrease it.
Q.15.Arrange the following compounds in decreasing order of their basic strength:
C6H5NH2, C2H5NH2, (C2H5)2NH, NH3
Ans. The decreasing order of the basic strength of above amines and ammonia is in following order:
(C2H5)2NH > C2H5NH2 > NH3 > C6H5NH2
Above order is due to aromatic amine is less basic than aliphatic amine and ammonia due to resonance stabilization of lone pair of nitrogen of aniline with benzene ring.
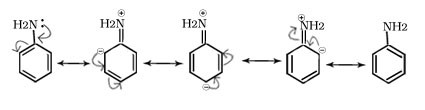
Q.16. –NH2 group in aniline is ortho and para directing towards electrophilic substitution reaction and a powerful activating group why?
Ans. Ortho- and para-positions to the –NH2 group become canters of high electron density due to +R (resonance). Thus –NH2 group is ortho and para directing and a powerful activating group.
Q. 17.The activating effect of –NHCOCH3 group is less than that of amino group.
Ans. The lone pair of electrons on nitrogen of acetanilide interacts with oxygen atom through resonance. Thus it is less available for donation to benzene ring by resonance. Therefore, activating effect of –NHCOCH3 group is less than that of the amino group.
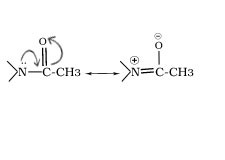
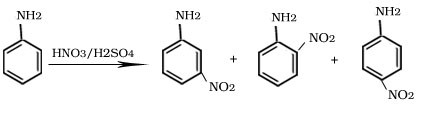
Q.18.Besides the ortho and para derivatives, significant amount of meta derivative is also formed during nitration of aniline why?
Ans. In the strongly acidic medium, aniline is protonated to form the anilinium ion which is meta directing.
Q.19.Aniline does not undergo Friedel-Crafts reaction (alkylation and acetylation) why?
Ans. Due to the salt formation with aluminum chloride, the Lewis acid, which is used as a catalyst, nitrogen of aniline acquires positive charge and hence acts as a strong deactivating group for further reaction.
Distinguish reactions
Distinguish between aliphatic primary amine/Aniline and secondary amine/tertiary amine.
Aliphatic primary amine/Aniline gives the carbylamines test but secondary amine/tertiary amine does not give this test.
R-NH2 + CHCl3 + 3KOH → R-NC + 3KCl + 3H2O (RNC is phenyl isocyanide which is foul smelling substance.
Distinguish between aliphatic primary amine and aniline.
Nitrous acid test is used for this test. Both aliphatic primary amine and aniline give this test but in different way.
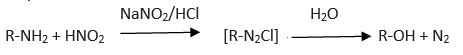
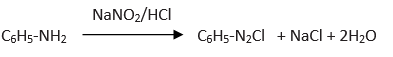
Benzene diazonium chlorides (C6H5N2Cl ) gives yellow color after coupling reaction with aniline.
Distinguish reaction between secondary amine and tertiary amine.
Hinsberg test is used for this reaction
Tertiary amines do not give this test. Secondary amines and primary amine react with Hinsberg reagent but product of primary amine and this reagent is soluble in KOH but that of secondary amine is insoluble.

Name Reactions
Ammonolysis
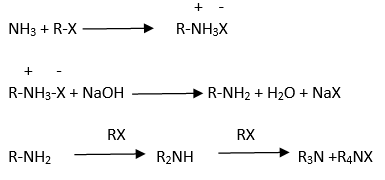
Gabriel phthalimide synthesis
Hoffmann bromamide degradation reaction

Coupling Reaction

Gattermann reaction
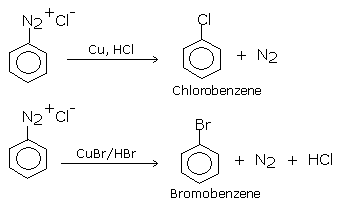
Other reactions
Acylation

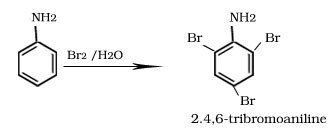
Bromination of aniline
Nitration of aniline
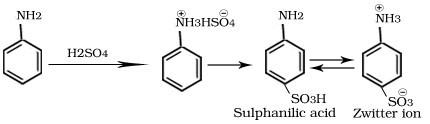
Sulphonation of aniline
Formation of amines from nitro compound

Formation of amine from amide
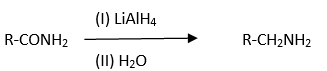
Formation of amine from nitriles
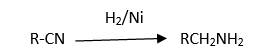
Reactions of Benzene diazonium chloride
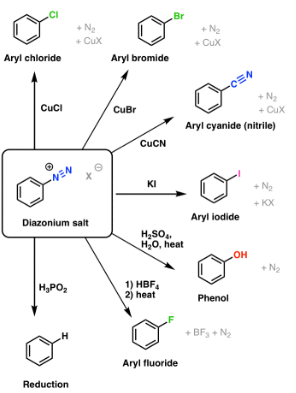
Formation of nitrobenzene from benzene diazonium chloride