Haloalkanes and Haloarenes
Reasoning based questions
Q.1.The reactions of primary and secondary alcohols with HCl require the presence of a catalyst, ZnCl2. With tertiary alcohols, the reaction is conducted by simply shaking with concentrated HCl at room temperature why?
Ans.It is due to order of reactivity of three classes of alcohols with HCl. Tertiary alcohols are most reactive hence do not need any catalyst. The order of reactivity of alcohols with a given haloacid is 3o>2o>1o
Q.2.The preparation of aryl halides is not possible from phenol and HX why?
Ans.Because the carbon-oxygen bond in phenol has a partial double bond character and is difficult to break being stronger than a single bond.
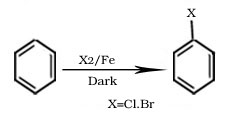
Q.3.Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride but aryl iodide and aryl fluoride is not possible by this method why?
Ans. Reactions with iodine are reversible in nature and require the presence of an oxidising agent (HNO3, HIO4) to oxidise the HI formed during iodination. Fluoro compounds are not prepared by this method due to high reactivity of fluorine.
Q.4.Boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
Ans.The chlorides, bromides and iodides are polar molecules and thus having dipole-dipole interaction between their molecules .On other hand hydrocarbons are non-polar molecules hence they contain weak dispersion forces .Thus boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
Q.5.The boiling points of alkyl halides decrease in the order: RI> RBr> RCl> RF why.
Ans. As the mass of the molecules increases size increases and thus, the magnitude of van der Waal forces increases hence boiling point increases.
Q.6.Arrange n-butyl bromide,isobutyl bromide,sec-butyl bromide and tertiary butyl bromide in decreasing order of boiling point.
Ans.
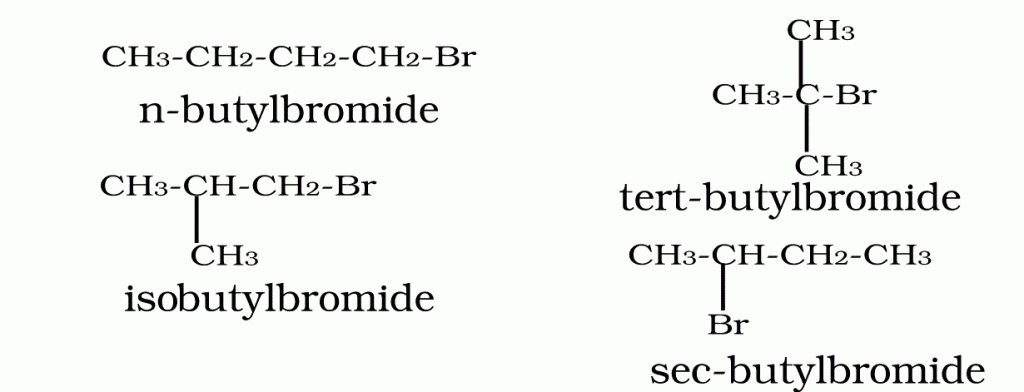
As branching increases surface area decreases hence magnitude of intermolecular vander waal forces decreases thus boiling point decreases.The order is-
n-butyl bromide > sec-butyl bromide > isobutyl bromide > tertiary butyl bromide.
Q.7. Arrange o-dichlorobenzene,p-dichlorobenzene, m-dichlorobenzene in decreasing order of their boiling and melting point.
Ans. o-dichlorobenzene > p-dichlorobenzene > m-dichlorobenzene (B.P).
p-dichlorobenzene > o-dichlorobenzene >m-dichlorobenzene (M.P).
The p-isomer has higher melting as compared to their ortho and meta-isomers. It is due to symmetrical structure of para-isomers that fits in crystal lattice better as compared to ortho– and meta-isomers.
Q.8. The density of alkyl halide increases with increase in number of carbon atoms why?
Ans. As the number of carbon atoms increases, mass increases thus density increases.
Q.9.The haloalkanes are only very slightly soluble in water why?
Ans. Haloalkanes molecules have dipole-Dipole interaction between their molecules, but water molecules have hydrogen bonding .Since hydrogen bonding is stronger force hence very less energy is realesed when new attraction is setup between haloalkanes and water molecules.
Q.10. Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Ans. (i) Chloromethane < Bromomethane < Dibromomethane < Bromoform
Above order is due to atomic mass which increases in same order.
(ii) 1-Chloropropane < Isopropyl chloride < 1-Chlorobutane.
1-Chlorobutane has highest molecular mass thus it has highest boiling point. Since Isopropyl chloride has more branching than 1-chloropropane hence former has lesser boiling than latter.
Q.11. Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanides as the chief product.
Ans. CN– is ambident nucleophile hence reaction can occur through C or N. Since KCN is ionic compound hence nucleophile CN– is available for bonding, since C-C bond is more stronger than C-N bond hence main product is cyanide. AgCN is covalent compound hence reaction occurs through N and main product is isocyanide.
Q.12.The order of reactivity towards S2N decreases in following order why?
Primary halide > Secondary halide > Tertiary halide
Ans.As the alkyl groups increases around carbon having leaving group it is getting very difficult to approach that carbon by nucleophile hence reactivity decreases in following order Primary halide > Secondary halide > Tertiary halide.
Q.13.S1N reactions are generally carried out in polar protic solvents (like water, alcohol, acetic acid, etc.).
Ans. In S1N mechanism 1st step involves the C–X bond breaking for which the energy is obtained through solvation of halide ion with the proton of protic solvent.
Q.15.Why is S1N reation 1st order and unimolecular reaction ?
Ans. In S1N mechanism 1st step involves the C–X bond breaking which is slow process and thus rate of reaction depends only on concentration of alkyl halide thus reation is 1st order and unimolecular.
Q.17. The order of reactivity towards S1N decreases in following order why?
Tertiary halide > Secondary halide > Primary halide
Ans.In S1N mechanism 1st step involves the C–X bond breaking and an intermediate carbocation forms. Since the stability of carbocation decreases in following order
3o˃2o˃1o (+I effect decreases)
Hence reactivity of alkyl halide decreases in following order Tertiary halide > Secondary halide > Primary halide.
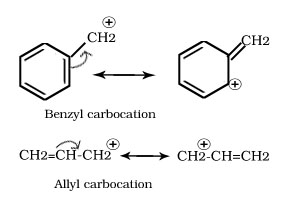
Q.18.Allylic and benzylic halides show high reactivity towards the S1N reaction.
Ans. The carbocation thus formed as intermediate gets stabilised through resonance.
Q.19.For a given alkyl group, the reactivity of the halide, R-X, follows the same order in both the mechanisms R–I> R–Br>R–Cl>>R–F why?
Ans.As the size of halogen increases bond dissociation energyof C-X decreases thus rate of releasing of leaving group decreases in following order I–˃Br–˃Cl–.Thus for a given alkyl group, the reactivity of the halide, R-X, follows the same order in both the mechanisms R–I> R–Br>R–Cl>>R–F.
Q.20.Grignard reagents should be prepared under anhydrous conditions?
R-MgX + H2O → R-H + MgOH(X). Grignard reagents are highly reactive in protic solvent like water , alcohol etc.
Q.21.Aryl halides are extremely less reactive towards nucleophilic substitution reactions why?
Ans.

Due to partial double bond character of C-Cl due to resonance , bond dissociation in haloarene is difficult than haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
Q.22.Although chlorine is an electron withdrawing group, yet it is ortho,para directing in electrophilic aromatic substitution reactions why?
Ans
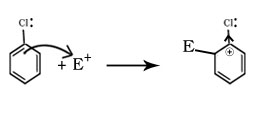
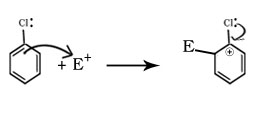
Cl on benzene ring produces two effects during electrophilic substitution one is inductive effect and other is resonance effect. By – I effect Cl decreases the stability of intermediate carbocation and by +R effect Cl increases the stability of intermediate carbocation. The inductive effect here is stronger than resonance and causes net electron deactivation and thus Cl is deactivating group.
Electron dcensity is high at ortho and para positions hence benzene ring donate electrons to the electrophile from these positions only hence Cl is ortho and para directing.
Q.23.Chloroform is stored in closed dark coloured bottles why?.
Ans,It is due to formation of poisonous gas phosgene.
2CHCl3 +O2 → 2COCl2 + 2HCl( COCl2 is phosgene)
Name reactions of Haloalkanes and Haloarenes
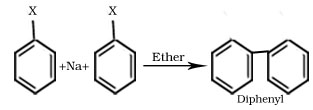
1.Wurtz reaction
R-X + 2Na + R-X → R-R + 2NaX
2. Sandmeyer’s reaction
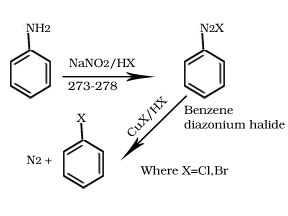
3. Finkelstein reaction
R-X + NaI → R-I + NaX
X=Cl, Br
4.Swarts reaction
H3C-X +AgF → H3C-F + AgBr(X=Cl, Br)
5. Friedel-Crafts alkylation reaction

6. Friedel -Craft acylation reaction

7 . Wurtz –Fittig reaction

8.Fittig reaction
Other reactions
Preparation of haloalakane from alcohol
- R-OH + H-X → R-X + H2O (ZnCl2 is used as catalyst when 1o and 2o alcohols are reacted with HCl)
2. R-OH + NaBr + H2SO4 → R-Br + NaHSO4 + H2O
3. R-OH + PX3 → R-X + H3PO3+ HCl (X=Cl,Br)
4. R-OH + PCl5 → R-Cl + R-X + POCl3 + HCl
5. R-OH + Red P/X2 → R-X (X=Cl2,I2)
6.R-OH + SOCl2 → R-Cl +SO2 + HCl
Formation of haloalkane from alkane
- CH3CH2CH2CH3 + Cl2/UV light → CH3CH2CH2CH2Cl + CH3CH2CHClCH3
2. Addition of H-X to alkene
Addition takes place according to markonikov rule,But addition of HBr in presence of peroxide takes place antimarkonikov rule.
CH2=CH2 +HX → CH3-CH2X
3.Addition of halogen to alkene
CH2=CH2 +Br2 /CCl4 → CH2Br -CH2Br
4.Formation of Grignard reagent
CH3-CH2-Br + Mg → CH3-CH2MgBr (Grignard reagent)
Formation of iodobenzene
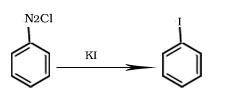
Formation of haloarene from benzene
Formation of phenol from chlorobenzene
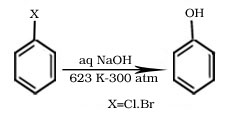
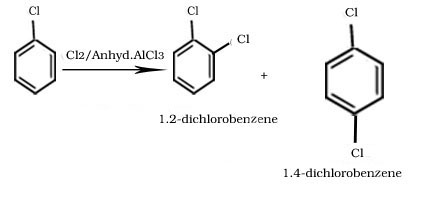
Halogenation Of Chlorobenzene
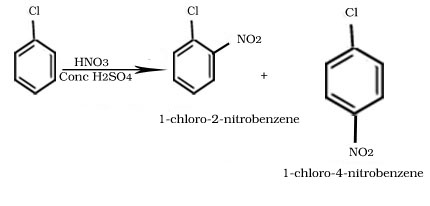
Nitration of Chlorobenzene
Sulphonation of Chlorobenzene

Mechanisms
SN1 Mechanism

1.SN1 mechanism completes in two steps.
2.In first step an intermediate carbocation forms and in second step nucleophile attacks the carbocation and formation of product can takes place.
3.Rate of reaction depends only on the first step and concentration of alkyl halide because it is slowest step thus SN1 is first order unimolecular reaction.
SN2 mechanism
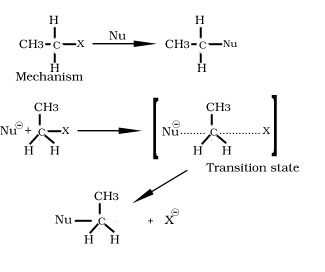
1 SN2 mechanism completes in one step.
2.A transition state forms where there is simultaneous attack of the nucleophile and displacement of the leaving group.
3. Rate of reaction depends both on concentration of nucleophile and alkyl halide thus reaction is 2nd order bimolecular reaction.
Sterochemistry involves during SN2 and SN1 mechanism
Q.1.What do you mean by optical activity of a particular compound?
Ans.Compound which rotate plane polarized light in either a clockwise or anticlockwise direction is called optical active compound.
Q.2.Define enantiomers.
Ans.Enantiomers possess identical chemical structures (i.e. their atoms are the same and connected in the same order), but are mirror images and nonsuperimposable of one another.They are optically active compounds one is called dextro (+) and another is called laevo(-) compound. Dextro rotate the plane polarised light in clockwise(right) direction and laevo in anticlockwise(left) direction.
Q.3.Define racemic mixture.
Ans. Mixture of equal amounts of dextro and laevo isomers results in no rotation called racemic mixture.racemic mixture is optically inactive.
Q.4.What is the criteria for optical activity?
Ans.A chiral compound is must be an optically active compound.
Q.5.What do you mean by chiral compound?
Ans. A compound which contain at least a chiral carbon(asymmetric carbonn) that is the carbon atom which is attached to four different types of atoms or four different groups of atoms that cannot be superimposed on their own mirror image are said to be chiral compound.
Q.6.Define retention ,inversion and partial racemisation.
Ans. Retention :The configuration of the molecule is retained through the reaction, so the product molecule has the same configuration as the reactant that is dextro reactant forms dextro product and laevo forms laevo.
Inversion: When the product’s configuration is opposite that of the substrate the process is called inversion.
Partial racemisation:The phenomena in which retention and inversion both takes place simultaneously called partial racemisation.
Q.7.Explain the sterochemistry involves during SN2 and SN1 mechanism.
Ans Experimental observation shows that all SN2 reactions proceed with inversion of configuration; that is, the nucleophile will always attack from the backside in all SN2 reactions. SN1 involves both retention and inversion that is it undergo partial racemisation configuration.
Polyhalogen Compounds
| Dichloromethane (Methylene chloride) Trichloromethane (Chloroform) Triiodo-methane (Iodoform) Tetrachloromethane (Carbon tetrachloride) Freons p,p-Dichlorodiphenyl trichloro ethane(DDT) | Cl2CH2 CHCl3 CHI3 CCl4 CCl2F2 CCl3CH(C6H5Cl)2 |
Uses of polyhalogen compounds
Cl2CH2
- Dichloromethane is widely used as a solvent as a paint remover.
- As a propellant in aerosols
- As a process solvent in the manufacture of drugs.
- It is also used as a metal cleaning and finishing solvent
CHCl3
- chloroform is employed as a solvent for fats, alkaloids, (Chloroform) the production of the freon refrigerant R-22
CHI3
- It was used earlier as an antiseptic but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself
CCl4
- It is produced in large quantities for use in the manufacture of refrigerants and propellants for aerosol cans.
- It is also used as feedstock in the synthesis of chlorofluorocarbons and other chemicals, pharmaceutical manufacturing.
CCl2F2
- These are usually produced for aerosol propellants, refrigeration and air conditioning purposes.
CCl3CH(C6H5Cl)2
- As insecticides
