Coordination compounds
werners theory of coordination compounds
- Metals have two type of linkages in coordination compounds one is called primary linkages and other is called secondary linkages.
- Primary linkage is equal the oxidation number of metal and secondary linkage is equal to the coordination number of the metal.
- The primary valences are ionisable and the secondary valences are non ionisable.
- Primary valences satisfied by negative ions and secondary valences are satisfied by neutral molecules.
- The secondary linkages of metal have characteristic spatial arrangements coordination polyhedra.
Q.1.Explain the primary linkage and secondary linkage with suitable example.
Ans. [Co(NH3)6]Cl3
In above compound three chlorides are primary linkages and 6 ammonia molecules are secondary linkages.
Q.2.Define counter ions.
Ans. Counter ions are simply the ‘simple’ cations or anions that may or may not participate in the Coordination. Ions outside the square bracket are called counter ions. In [Co(NH3)6]Cl3 , [Co(NH3)6]3+ is coordination entity and 3Cl– are counter ions.
Q.3.What is the difference between double salt and complex salt.
Ans.Double salt dissociates into simple ions completely when dissolved in water.Example, Carnallite, KCl.MgCl2.6H2O dissociates into K+,Cl– and Mg2+.Complex salt dissociates into simple ion and complex ion for example K4Fe(CN)6 dissociate into complex ions [Fe(CN)6]4– and k+, [Fe(CN)6]4– do not dissociate into Fe2+ and CN– ions.
Q.4.Define coordination entity.
Ans.Central metal atom and secondary linkages are collectively called coordination entity. For example [CoCl3(NH3)3]3+ is a coordination entity in which the cobalt ion is surrounded by three ammonia molecules and three chloride ions.
Q.5.Define central atom/ion.
Ans.In coordination entity the metal which is bound with fixed number of secondary linkages is called central atom. For example [CoCl3(NH3)3]3+ is a coordination entity in which the cobalt ion is central ion.
Q.6.Define ligand.
Ans.The negative ions and neutral molecules which are bound to the central atom as secondary linkages are celled ligand, for example [CoCl3(NH3)3]3+ is a coordination entity in which three ammonia molecules and three chloride ions are ligands.
Q 7.Explain the types of ligand.
Ans.Ligands are of two types.
1.Monodentate or unidentate ligand.
When a ligand is bound to a metal ion through a single donor atom is called unidentate ligand For example in [CoCl3(NH3)3] Cl and NH3 are unidentate ligand.
2.Polydentate ligand.
When a ligand is bound to a metal ion through more than one donor atoms is called polydentate ligand for example in [Co(en)2(Cl)2], ‘en’ is polydentate ligand.
Polydentate ligand can further divided into didentate,terdentate,tetradentate ,pentadentate ,hexadentate etc.
Q.8.Define didentate ligand.
Ans. When a ligand is bound to a metal ion through two donors atoms is called bidentate ligand for example in [Co(en)2(Cl)2], en is didentate ligand.
Q.9.Give an example of hexadentate ligand.
Ans. Ethylenediaminetetraacetate ion (EDTA4–) is hexadentate ligand.
Q.10.Define ambidentate ligand.
Ans. Ligand which can coordinate with central atom through two different atoms is called ambidentate ligand.For example;
NO2– It can coordinate either through nitrogen or through oxygen to a central metal atom/ion.
SCN– It can coordinate through the sulphur or nitrogen atom to a central metal atom/ion.
Q.11.Define chelation and chelate effect.
Ans.The phenomena in which polydentate ligand is coordinate with central atom.The stability of complex compound increases this effect is called chelate effect.
Q.12.Define coordination number.
Ans. The number of ligand donor atoms to which the metal is directly bonded is called coordination number for example in [Co(en)2(Cl)2], ‘Co’ has coordination number 6 because ‘en’ is didentate ligand.
Structure of complex compounds on the basis of coordination number
| Coordination number | Structure |
| 2 | linear |
| 3 | Triagonal |
| 4 | Tetrahedral or square planer |
| 5 | Pentagonal bipyramial |
| 6 | Octahedral |
Q.13.Define homoleptic and heteroleptic complexes?
Ans. Complexes in which a metal is bound to only one kind of donor groups are known as homoleptic for example [Co(NH3)6]3+.
Complexes in which a metal is bound to more than one kind of donor groups, are known as heteroleptic for example [Co(NH3)4Cl2]+
List of unidentate and didentate ligands
| Name | Symbol | Charge | IUPAC Name |
| fluoride | F | -1 | -e+o =fluorido |
| chloride | Cl | -1 | -e+o =chlorido |
| bromide | Br | -1 | -e+o =bromido |
| iodide | I | -1 | -e+o =iodido |
| Hydroxide | OH | -1 | -ide+o=hydroxo |
| Cyanide | CN | -1 | -ide+o=cyano |
| Nitrite | NO2 | -1 | -e+o =nitrito |
| Sulphate | SO4 | -2 | -e+o =sulphato |
| Carbonate | CO3 | -2 | -e+o =carbonato |
| Water | H2O | 0 | aqua |
| Ammonia | NH3 | 0 | ammine |
| Carbonmonooxide | CO | 0 | carbonyl |
| Primary amine | R-NH2 | 0 | Alakananine |
| triphenylphosphine | PPh3 | 0 | triphenylphosphine |
| Thiocyanate | SCN | -1 | -e+o =thiocyanato |
| nitric oxide | NO | 0 | nitrosyl |
| nitric oxide | NO | +1 | Nitrosonium |
| nitric oxide | NO | -1 | Nitroso |
| Isothiocyanate | NCS | -1 | Isothiocyanato |
List for bidentate ligand.
| Name | Symbol | Charge | IUPAC Name |
| Ethylenediamine | en, (NH2-CH2-CHNH2) | 0 | Ethane-1,2-diamine |
| Oxalate | Ox, (C2O42-) | -2 | -e+o=Oxalato |
Rules for the IUPAC Nomenclature of coordination compounds
Complex compounds are of three types.
| Coordination compounds with +ve coordination entity | Coordination compounds with -ve coordination entity | Coordination compound with neutral coordination entity |
| [Co(NH3)4Cl2]Cl Rule. 1.Name the positive part first. Since in above example +ve part is complex ion hence name the +ve part by following rules. a.Name the ligands and arrange all in alphabetical order,use di,tri ,tetra etc if same type of ligand is present at more than one times. tetraamminedichlorido b.Name the central atom with its oxidation number in ( ) bracket. Tetraamminedichloridocobalt(III) 2.Name the negative part. Here –ve part is simple ion . Tetraamminedichloridocobalt(III) Chloride. Point to be noted a. When the name of the ligands include di tri etc, then use, bis(for 2), tris(for 3)and tetrakis(for 4) . b.There is gap between +ve and –ve part. c.For ambidentate ligand NO2 use ‘N’or O before the name of central atom depending from which atom ligand is coordinated. For example [Pt(NH3)BrCl(NO2)]– Amminebromidochloridonitrito-N-platinate(II) and for [Pt(NH3)BrCl(ONO)]– Amminebromidochloridonitrito-O-platinate(II) | K4[Fe(CN)6] Rule. 1.Name the positive part first. Potassium 2.Name the negative part. Since in above example -ve part is complex ion hence name the -ve part by following rules. a.Name the ligands and arrange all in alphabetical order,use di,tri ,tetra etc if same type of ligand is present at more than one times. hexacyano b. Name the central atom with its oxidation number in ( ) bracket. c.use –ate as suffix in the name of central atom Potassium hexacyanoferrate(II) Point to be noted a. When the names of the ligands include di tri etc, then use, bis(for 2), tris(for 3)and tetrakis(for 4) . b.There is gap between +ve and –ve part. c. For some metals, the Latin names are used in the complex anions, e.g., ferrate for Fe, argentate for Ag | [Ni(CO)4] Name it in single word. a.Name the ligands and arrange all in alphabetical order,use di,tri ,tetra etc if same type of ligand is present at more than times. tetracarbonyl b. Name the central atom with its oxidation number in ( ) bracket. Tetracarbonylnickel(0) |
Formula for mononuclear coordination entities(contain a single central metal atom)
We start with example tris(ethane-1,2–diammine)cobalt(III) sulphate
1.Write the central atom first in [ ] bracket
[Co]
2.Write all ligands after central atom
[Co(en)3]
3.Write the counter ion [Co(en)3](SO4)
4.Since we do not know the number of +ve ion and –ve ion so let them x and y respectively.
[Co(en)3]x(SO4)y
Now find the value of x and y by using oxidation number of central atom.
3+0.x+(-2)y=0
Hence 3x=2y
Hence x=2 and y=3
Thus compound is [Co(en)3]2(SO4)3
Types of structural isomers with example
Structural isomers have 4 types.
1.Linkage isomesrs.
[Co(NH3)5(NO2)]Cl2 & [Co(NH3)5(ONO)]Cl2
Linkage isomers are formed by ambidentate ligand .In above example NO2 is ambidentate ligand ,forms two isomers. One is formed when central atom is bound through nitrogen and other when it is bound through oxygen.
2.Coordination isomer:
[Cr(NH3)6][Co(CN)6] &[Co(NH3)6][Cr(CN)6]
Coordination isomers are formed when ligands are interchanged between cationic and anionic entities of different metal ions present in a complex.
3.Ionisation isomers.
[Co(NH3)5SO4]Br & [Co(NH3)5Br]SO4
Ionisation isomers are formed when counter ion and ligands interchange their position. counter ion becomes ligand and replace a ligand which becomes counter ion.
4.Hydrate isomers or solvate isomers.
[Cr(H2O)6]Cl3 and[Cr(H2O)5Cl]Cl2.H2O
Hydrate isomers are similar with ionization isomers.It involves solvent molecules like water,water molecules are bound with central atom as ligand or present as free solvent molecules.
Geometrical isomers of coordination compounds
Complex compounds having coordination number either 4(Square planer) or 6 (octahedral) can show geometrical isomerism
Types.
[MA2B2]
[MA2BC]
[MA2B4]
[MA4BC]
[MA2X2]
[MA2B2X]
[MABX2]
[MABCD]
[MA3B3]
[MABCDEF]
Where ABCD are monodentae ligand and X is bidentate ligand.
Structure of geometrical isomers of complex compound?
[MA2B2]

[MA2BC]
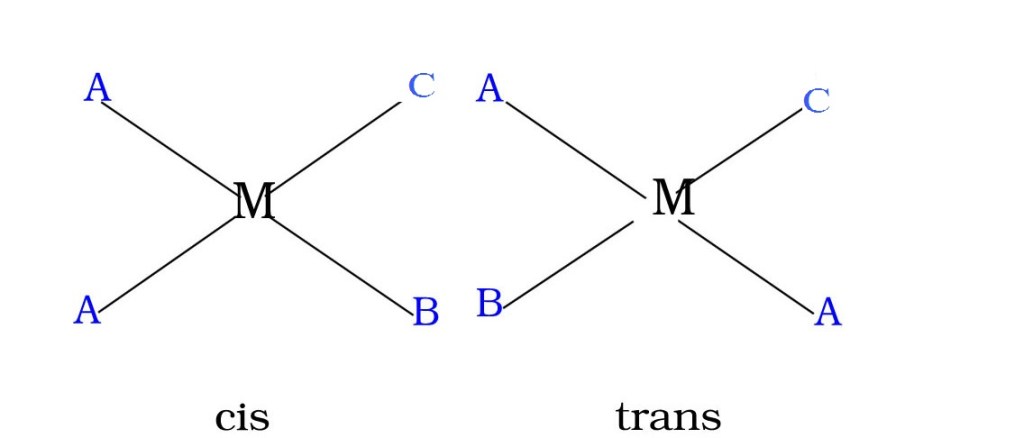
[MA2B4]

[MA4BC]
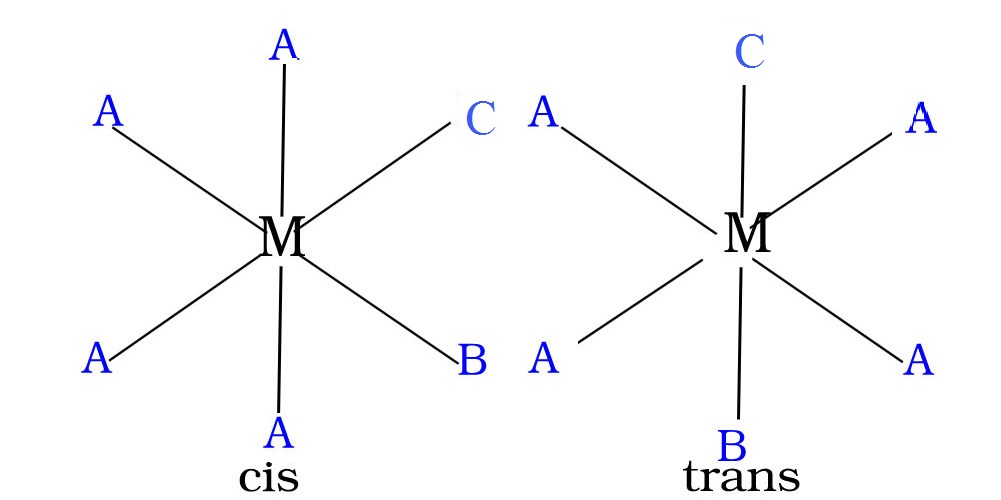
[MA2X2]

[MABX2]

[MA2B2X]

[MABCD]

[MA3B3]

Where ABCD are monodentae ligand and X is bidentate ligand.
Conditions to show optical isomers by complex compound
Complex compounds having coordination number 6(octahedral) can give optical isomers. Very few tetrahedral complexes show optical isomerism.Square planer complexes are seldom optically active.
Types.
[MA2X2]
[MA2B2X]
[MABX2]
[MX3]
Where A,B are monodentae ligand and X is bidentate ligand.
Structures of all types of optical isomers
[MA2X2]

[MA2B2X]

[MX3]
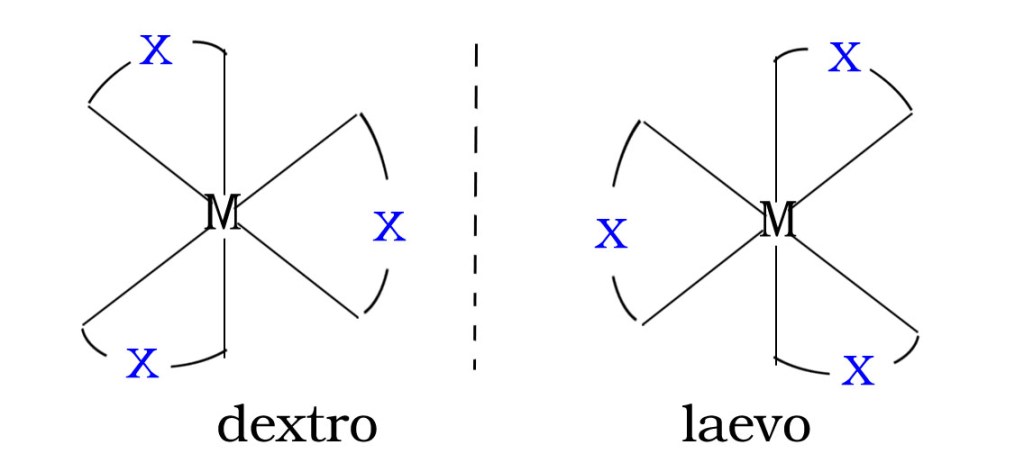
[MABX2 ]
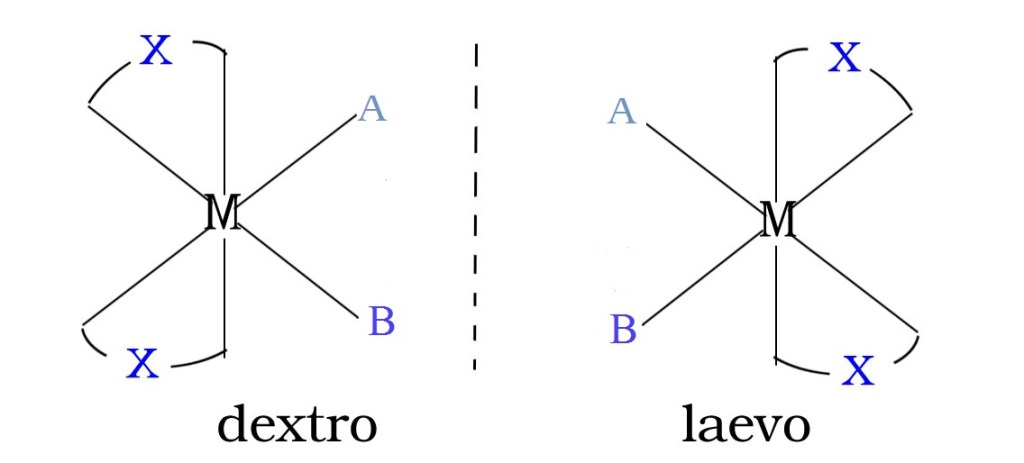
Valence Bond theory of coordination compounds
We take a example [CoF6]3–
Step 1.Write the electronic configuration of central atom.

Step 2.Calculate oxidation number of central atom.
X+(-1)6=-3
X=3
Step 3. .Write the electronic configuration of central atom ion.

Step 4. Write the electronic configuration of [CoF6]3–
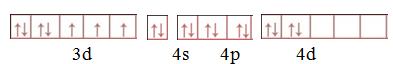
Hybridiastion is sp3d2
Structure –octahedral
Magnetic property-Paramagnetic. (due to presence of unpair electron)
Behavior of complex-Outer orbital complex .
Structure ,magnetic property and color of the complex compound according to VBT if ligand is strong like CO and CN.
We take a example [Co(CN)6]3–
Step 1.Write the electronic configuration of central atom.

Step 2.Calculate oxidation number of central atom.
X+(-1)6=-3
X=3
Step 3. .Write the electronic configuration of central atom ion.

Step.4.Pairing of d electrons take place.
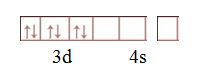
Step 5. Write the electronic configuration of [CoF6]3–
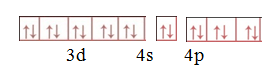
Hybridiastion is d2sp3
Structure –octahedral
Magnetic property-Diamagnetic. (Due to presence of paired electrons)
Behavior of complex-Inner orbital complex .
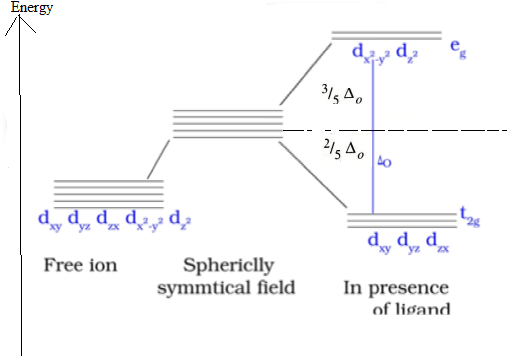
CFT (Crystal field theory)
The five d orbitals have same energy in isolated gaseous atom and in spherically symmetrical field that is, when –ve ion surrounds the metal hence they are called degenerate orbital.This degeneracy is disturbed in presence of ligand.Thus this splitting of the d orbitals in presence of ligand is called crystal field theory.
d orbital splitting diagram in an Octahedral crystal field
d orbital splitting diagram in an tetrahedral field
Energy

Q.14 What is spectrochemical series?
Ligands are arranged in the order of increasing field strength is called spectrochemical series.
I– < Br– < SCN– < Cl– < S2– < F – < OH– < C2O4 2– < H2O < NCS– < edta4– < NH3 < en < CN– < CO
Q.15 Define strong field ligand and weak field ligand.
Strong and weak field ligands. The spectrochemical series ranks ligands according the energy difference ΔO between the t2g and eg orbitals in their octahedral complexes. This energy difference is measured in the spectral transition between these levels, which often lies in the visible part of the spectrum and is responsible for the colors of complexes with partially filled d-orbitals. Ligands that produce a large splitting are called strong field ligands, and those that produce a small splitting are called weak field ligands.
Q.16 What is crystal field splitting energy? How does the magnitude of Δ0 decide the actual configuration of d-orbitals in a coordination entity?
Ans.When the ligands approach a transition metal ion, the d-orbitals split into two sets, one with lower energy and the other with higher energy. The difference of energy between the two sets of orbitals is called crystal field splitting energy.
Let p= The energy required for electron pairing in a single orbital.
Δ0 =Energy difference between t2g and eg set of orbitals in octahedral complex.
Strong field ligand– When Δ0 >P,The fourth electron occupies a t2g orbital with configuration t2g4eg0
Weak field ligand– When Δ0 <P The fourth electron enters one of the eg orbitals giving the configuration t2g3eg1
Q.17 Explain bonding in metal carbonyl compound with example.
Ans.The metal-carbon bond in metal carbonyls possess both ![]() and
and ![]() bond .The
bond .The![]() is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. The
is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. The ![]() is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding
is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding ![]() orbital of carbon monoxide example Ni(CO)4 .
orbital of carbon monoxide example Ni(CO)4 .
Q.18.Why are some of the coordination compounds colored?
Ans. Partly filled d orbitals complex having transition metal as central atom can show colour.Electrons of lower d orbitals absorbed color in visible region and go to the higher state d orbitals.The color of the complex is complementary color of the light absorbed.
Q.19 How is stability of coordination compound determined?
Ans .Stability of coordination compound is determined by stability constant.
Suppose a reaction type ![]() stability constant is
stability constant is ![]() . Higher the value of
. Higher the value of ![]() higher the stability of coordination compound.
higher the stability of coordination compound.
Q.20.Define instability constant or dissociation constant.
Ans.The reciprocal of the association constant is called instability constant or dissociation constant .
Q.21.What are the uses of coordination compound?
Ans.
- EDTA, DMG(dimethylglyoxime), are used in qualitative and quantitative chemical analysis.
- In determining of hardness of water Na2EDTA is used.
- The pigment responsible for photosynthesis, chlorophyll, is a coordination compound of magnesium.
- Haemoglobin is a coordination compound of Iron.
- Vitamin B12, cyanocobalamine, the anti–pernicious anaemia factor, is a coordination compound of cobalt.
- Carboxypeptidase A and carbonic anhydrase are used as enzyme catalyst.
- Wilkinson catalyst (rhodium complex, [(Ph3P)3RhCl]) is used for the hydrogenation of alkenes.
- EDTA is used in the treatment of lead poisoning.
- cis–platin and related compounds inhibit the growth of tumours.
