d-and f-block elements
Q.1.Why are d-block elements called transition elements?
Ans.The position of d-block elements in the periodic table is between s-block elements and p-block elements. They exhibit transitional behaviour between s-block and p-block elements,so they are known as transition elements.
Q.2.Write different types of transition elements.
Ans.There are mainly three types of transition series.
1.1st transition series- 3d series (Sc to Zn).
2. 2nd transition series-4d series (Y to Cd)
3. 3rd transition series-5d series (La to Hg)
Q.3.Zinc, cadmium and mercury are not considered as transition elements why?
Ans.They have completely filled d orbital(d10) in their ground state as well as in their oxidation state(M2+) hence they are not considered as transition elements.
Q.4.What is the general electronic configuration of d-block elements?
Ans.(n-1)d1–10ns1–2 where n is outermost shell.
Q.5.Write the electronic configuration of Cr and Cu.
Ans.Cr(24)- [Ar] 3d5 4s1
Cu(29)-[Ar] 3d104s1
Q.6.Transition metals are hard ,have high melting points and have low volatility why?
Ans. Transition metals have strong interatomic metallic bonding due to the involvement of greater number of electrons from (n-1)d in addition to the ns electrons because the energy difference between ns electrons and (n-1)d electrons is very small.
Q.7.Zn, Cd and Hg are not hard why?
Ans.They have completely filled d orbital(d10) hence the interatomic metallic bonding is weak. Thus Zn, Cd and Hg are not hard.
Q.8.In any row of transition series the melting points of transition elements rise to a maximum at middle except for anomalous values of Mn and Tc and fall regularly as the atomic number increases why?
Ans.The strength of interatomic metallic bonding increases and becomes maximum at middle(d5) because number of unpair electron increases up to middle. From middle to end strength of metallic bonding decreases as number of unpair electron decreases. Thus in any row the melting points of transition elements rise to a maximum at middle except for anomalous values of Mn and Tc and fall regularly as the atomic number increases.
Q.9.Enthalpies of atomisation of transition elements is very high why?
Ans.It is due to the presence of large number of unpaired electrons in their atoms they have strong interatomic metallic bonding and hence higher enthalpies of atomisation.
Q.10.In 1st transition series the enthalpy of atomization of zinc is the lowest why?
Ans. Zinc have completely filled d orbital(d10) hence the interatomic metallic bonding is weak.
Q.11.Atomic and ionic size of of transition element decreases with increasing atomic number why?
Ans. As atomic number increases the new electron enters a d orbital each time the nuclear charge increases by unity and also the shielding effect of a d electron is very low,hence the net electrostatic attraction between the nuclear charge and the outermost electron increases and the ionic /atomic radius decreases.
Q.12.The variation in atomic /ionic size within a series is quite small why?
Ans.It is due to the presence of d electrons and shielding effect of these electrons is very low.
Q.13.Define lanthanoid contraction.
Ans.The filling of 4f before 5d orbital results in a regular decrease in atomic radii called lanthanoid contraction which essentially compensates for the expected increase in atomic size with increasing atomic number
Q.14.The radii of the 3rd (5d) series are nearly same with those of the corresponding members of the 2nd series.
Ans.It is due to lanthanoid contraction. The filling of 4f before 5d orbital results in a regular decrease in atomic radii called lanthanoid contraction which essentially compensates for the expected increase in atomic size with increasing atomic number.
Q.15.Zr and Hf have very similar physical and chemical properties why?
Ans.Zr and Hf have similar atomic radii due to lanthanoid contraction. The filling of 4f before 5d orbital results in a regular decrease in atomic radii called lanthanoid contraction which essentially compensates for the expected increase in atomic size with increasing atomic number.
Q.16.There is an increase in ionisation enthalpy along each series of the transition elements from left to right why?
Ans. Atomic and ionic size of transition element decreases with increasing atomic number due to poor shielding effect d electrons.
Q.17.Variation in ionization enthalpies of transition element is small why?
Ans. Because variation in atomic /ionic size within a series is quite small.
Q.18.Zinc has high 1st ionization enthalpy why?
Ans.Because electron is removing from stable 4s2 configuration.
Q.19.Zinc has lower 2nd ionization enthalpy.
Ans.After removal of an electron from 4s1 electronic configuration of d orbital remain unchanged.
Q.20.What is the lowest common oxidation state of elements of 1st transition series.
Ans.The lowest common oxidation state of elements of 1st transition series is +2 when 4s electrons are used.
Q.21.Second ionisation enthalpy of Cr and Cu has unusual high values why?
Ans.After removal of one electron from Cr and Cu it converts into stable Cr+(3d5)configuration and Cu+(3d10) configuration respectively hence second ionisation enthalpy of Cr and Cu has unusual high values.
Q.22.The third ionization enthalpy of Mn is high why.
Ans After removal of 2 electrons from Mn it converts into stable Mn2+(3d5)configuration.
Q.23.Transition elements show variable oxidation state why?
Ans.Transition elements have large number of unpair electrons and also these elements use not only ns electrons but also( n-1)d electrons because energy difference between ns and (n-1)d electrons are very small.
Q.24.Scandium does not exhibit variable oxidation states why?
Ans .Sc has only one electron in d orbital hence it can show only one oxidation state +3.
Q.25.Manganese shows largest number of oxidation states that is from +2 to 7 why?
Ans. Mn atoms have maximum number of unpaired electrons (3d5 configuration).
Q.26.The first and second ionization enthalpies in the first series of the transition elements are irregular why?
Ans.Ionisation enthalpy depends not only on the size of elements but also stability of electronic configurations .Some electronic configurations like d0.d5,d10 have extra stability hence the first and second ionization enthalpies in the first series of the transition elements are irregular.
Q.27.The E0(Cu2+/Cu) value for copper is positive (+0.34V) why?
Ans. E0 value depends on following factors.
i.Enthalpy of atomization.
ii.Ionisation enthalpy.
iii.Hydration enthalpy.
Since enthalpy of atomization of Cu is high and hydration enthalpy of Cu2+ is low hence, the E0(Cu2+/Cu) value for copper is positive (+0.34V).
Q.28.Cu is not able to liberate H2 from acids why?
Or
Only oxidising acids (nitric and hot concentrated sulphuric) react with Cu.
Ans. The E0 (Cu2+/Cu) value for copper is positive (+0.34V) due to low hydration enthalpy of Cu2+ and high enthalpy of atomization of Cu.
Q.29.E0value for Mn, Ni and Zn are more negative than expected from the trend why?
Ans.It is due to stable half filled electronic configuration of Mn2+ and fully filled stable electronic configuration of Zn2+ and high hydration enthalpy of Ni2+.
Q.30.Mn3+ is oxidising in nature but Cr2+ reducing in nature why?
Ans.Both Mn3+and Cr2+ have d4 configuration but Mn3+ is reduced into Mn2+ (from d4 to d5) and Cr2+ is oxidized into Cr3+ (from d4 to d3).Both Mn2+ and Cr3+ are stable due to different reasons. Mn2+ has stable half filled d5 configuration and Cr3+ has stable half filled t2g configuration,hence Mn3+ is oxidising but Cr2+ reducing in nature.
Q.31.E0(M3+/M2+)value for Mn is high why?
Ans.It is due to high ionization enthalpy is required to remove an electron from half filled stable electronic configuration of Mn2+( d5), E0(M3+/M2+) value for Mn is high.
Q.32.Copper (I) compounds are unstable in aqueous solution and undergo disproportionation?
Ans.
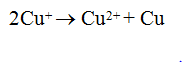
 of Cu2+ is higher than that of Cu+ hence 2nd ionization enthalpy of cu is much more compensated by hydration enthalpy of Cu2+ .
of Cu2+ is higher than that of Cu+ hence 2nd ionization enthalpy of cu is much more compensated by hydration enthalpy of Cu2+ .
Q.33.The ability of oxygen to stabilise higher oxidation states exceeds that of fluorine why?
Or
The highest Mn fluoride is MnF4 whereas the highest oxide is Mn2O7 why?
Ans. It is due to ability to form multiple bonds by oxygen atom.
Q.34.Compare reducing strength between Cr2+ and Fe2+.
Ans.
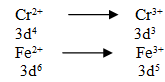
since 3d3 configuration is more stable than 3d5 configuration in aq solution hence E0 value of Cr3+/Cr2+ is lesser than Fe3+/Fe2+ thus Cr2+ is more reducing than Fe2+.
Q.35.In the first row transition metals show irregular values of Eo(M2+/M) as we go from left to right why?
Ans. E0 value depends on following factors.
i.Enthalpy of atomization.
ii.Ionisation enthalpy.
iii.Hydration enthalpy
Since first and second ionization enthalpies in the first series of the transition elements are irregular and sublimation enthalpy of manganese and vanadium is much less hence the first row transition metals show irregular values of Eo(M2+/M) as we go from left to right.
Q.36.E0(Mn3+/Mn2+) value is more positive than Cr3+/Cr2+ or Fe3+/Fe2+?
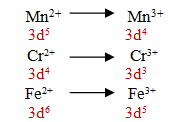
Due to very high ionization enthalpy in removal of an electron from half filled stable electronic configuration of Mn2+( d5) E0(Mn3+/Mn2+) value is more positive than Cr3+/Cr2+ or Fe3+/Fe2.
Q.37.The highest oxidation state of a metal present in its oxide or fluoride only why?
Ans.It is due to high electronegativity of oxygen and fluorine which can stabilize the large oxidation state of metal.
Q.38.Many of the transition metal ions are paramagnetic why?
Ans.Most transition element ions having a magnetic moment due to presence of unpaired electrons hence they are paramagnetic.
Q.39.Sc3+ and Zn2+ are diamagnetic why?
Ans. These ions have no magnetic moment due to presence paired electrons hence they are diamagnetic.
Q.40.What is ‘spin-only’ formula and how magnetic moment is determined by this formula.
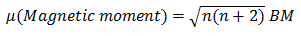
n= number of unpaired electrons.Higher the number of unpair electrons,higher the magnetic moment and thus higher the paramagnetism.
Q.41.Why are transitional metal ions coloured?
Ans.It is due to d-d transition ,in presence of ligands splitting of d orbitals are occurred and electrons of lower d orbitals are excited to higher d orbitals by absorbing light in visible region,and thus new color of solution is complementary colour of the light absorbed.
Q.42.Sc3+ and Zn2+ are colorless why?
Ans. d-d transition is not possible on both cases because Sc3+ has no d electron and Zn2+ has completely filled d orbitals.
Q.43.The transition metals form a large number of complex compounds why?
Ans.It is due to following reasons.
i. Smaller sizes of the metal ions.
ii. High ionic charges.
iii.Availability of d orbitals for bond formation.
Q.44.The transition metals are good catalyst why?
Ans.It is due to the following reasons.
i. During catalytic reaction they can adopt multiple oxidation states.
ii.They can form complexes.
Q.45.Give examples of some reactions in which transition metal can acts like catalyst.
Ans. I. Vanadium(V) oxide in contact Process for manufacture of sulphuric acid.
II. Finely divided iron in Haber’s Process for manufacturing ammonia.
III. Nickel in Catalytic Hydrogenation of alkenes.
Q.46.Define interstitial compounds.
Ans.A compound of transition metals and hydrogen, carbon ,nitrogen etc when these smaller metals are trapped into interstitial of crystal lattice.
Examples: example, TiC, Mn4N, Fe3H
Q.47.What are the properties of interstitial compounds.
(i) High melting points
(ii) Very hard.
(iii) Metallic conductor.
(iv) Chemically inert.
Q.48.Explain disproportionation reaction with example .
Ans.In a particular reaction when same compound is reduced and oxidized both, the reaction is called disproportionation reaction.

In above example oxidation number of Mn increases from 6 to 7 and also decreases from 6 to 4.
Q.49.Inner transition elements are called f-block elements why?
Ans. The last electron of these elements enters in the ‘f’’ orbital that is why they are called are called f-block elements
Q.50.Why f-block elements are called inner transition elements.
Ans d-block elements are called transition elements and their two shells
(last and the penultimate shells)are incomplete and since last electrons of f-block elements are filled in f orbitals which are inner orbitals of penultimate shell that is why f-block elements are called inner transition elements.
Q.51.How many types of f-block elements.
Ans. Lanthanoids (The fourteen elements after lanthanum).
Actinoids (the fourteen elements after actinium).
Q.52.General electronic configuration of f-block elements.
Ans.(n-2)f0-14 (n-1)0-1ns2
Where n is outermost shell.
For Lanthanoids n=6
For Actinoids n=7
Q.53.What is the common oxidation state of lanthanoids.
Ans. +3 is the common oxidation state of lanthanoids.
Q.54.The first and second ionization enthalpies of the innertransition elements are irregular why?
Ans.It is due to the extra stability of f0,f7,f14 electronic configuration.
Q.55.Ce can exhibit +4 oxidation state why?
Ans.Due to noble gas electronic configuration of Ce in +4 oxidation state it can show this oxidation state.
Q.56.Name the lanthanoids ions which do not show paramagnetic behaviour.
Ans. La3+ and Ce4+(due to f0 configuration)
Yb2+ and Lu3(due to f14 configuration)
Q.57. Name the lanthanoids ions which do not show color in aq solution.
Ans. La3+ and Lu3+ due to f0 and f14configuration configuration respectively.
Q.58.Most of the trivalent lanthanoid ions are coloured both in the solid state and in aqueous solution why?
Ans.Due to presence of unpair f electrons in these trivalent ions they show colour.
Q.59.Explain the abnormally low value of the third ionisation enthalpy of lanthanum, gadolinium and lutetium in lanthanoids series of elements.
Ans.It is due to half filled configuration (f7) of La3+ and Gd3+ and fully filled configuration (f14) of Lu+3.
Q.60.What are the uses of lanthanoids.
Ans. A lanthanoid metal (almost 95%), iron (almost 5%) and traces of S, C, Ca and Al are used to make an alloy named mischmetall. Mischmetall is used in Mg-based alloy to produce bullets, shell and lighter flint.
Q.61.Are all the actinoids are radioactive elements.
Ans, Yes all actinoids are radioactive elements.
Q.62.Define actinoid contraction.
Ans.The gradual decrease in the size of atoms or M3+ ions across the actinoids series is called actinoid contraction.
Q.63.Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Ans.In lanthanoids electrons are filled in 4f orbitals but in actinoids electrons are filled in 5f orbitals since shielding effect of 5f electrons are lower than 4f electrons hence actinoid contraction is greater from element to element than lanthanoid contraction.
Q.64.Why actinoids show greater range of oxidation in comparision with lanthanoids .
Ans.The energy difference between 5f, 6d and 7sorbitals in case of actinoids are lesser than that of 4f ,5d and 6s orbitals in case of lanthanoids hence actinoids show greater range of oxidation in comparison with lanthanoids .
Q.65.Describe the reactivity of actinoids.
Ans.1 All actinoids are highly reactive.
2.The metal readily tarnish in air.
3.They can react with boiling water and dilute acids and can liberate hydrogen gas.
4.They form oxides and hydrides and other products when combine with nonmetals dircetly.
