The p-block elements
Q.1.What is the position of p- block elements in periodic table?
Ans.p-block elements are placed in right of the periodic table. Group 13-18 elements are p-block elements.
Q.2.What is the general electronic configuration of the last shell of p-block elements?
Ans. ns2np1-6 .
Group 15 elements.
News Paper As Sb Bik gaya(N, P, As Sb Bi)
Q.1.Arrange atomic size of group 15 elements in inreasing order.
Ans.N< P< As < Sb < Bi.(One shell increases per period when we go top to bottom in group.)
Q.2.Arrange 1st ionization enthalpy of group 15 elements in decreasing order.
Ans. N> P >As > Sb > Bi(Atomic size increases top to bottom in group)
Q.3. Arrange negative electron gain enthalpy of group 15 elements in increasing order.
Ans. N> P >As > Sb > Bi(Atomic size increases top to bottom in group)
Q.4. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed why.
Ans.It is due to the poor shielding effect of d and f electrons which are present in heavier members.
Q .5. The ionisation enthalpy of the group 15 elements is much greater than that of group 14 elements in the corresponding periods Why?
Ans. Group 15 elements are smaller in size with respect to group 14 elements in the corresponding periods and also group 15 elements have stable half-filled p orbitals.
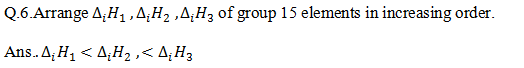
Q.7. Arrange electronegativity of group 15 elements in increasing order.
Ans. N> P >As > Sb > Bi (Atomic size increases top to bottom in group)
Q.8.Explain the physical state of group 15 elements.
Ans. Dinitrogen is gas while all others elements are solids.
Q.9.Explain the metallic property of group 15 elements.
Ans. N- Non metal.
P- Non metal.
As-Metalloids.
Sb-Metalloids
Bi-Metal.
Thus we can say that metallic properties increases top to bottom in group. It is due to ionization enthalpy decreases top to bottom.
Q.10. The melting point increases up to arsenic and then decreases up to bismuth why?
Ans.It is due to atomic size increases from N –As then metallic character increases.
Q.11.The boiling points, in general, increase from top to bottom in the group but Bi has lower boiling point than Sb why?
Ans This is due to interatomic attraction which is lower in Bi.
Q.12.Explain allotropic nature of group 15 elements.
Ans.Except Bi, all the elements show allotropic nature.
Q.13.What is the oxidation range of group 15 elements.
Ans.-(8-group no)- Group no. thus range is –(8-5) to 5 that is -3 to 5.
Q.14.What are the common oxidation states group 15 elements.
Ans. –3, +3 and +5.
Q.15. The tendency to exhibit –3 oxidation state decreases down the group why?
Ans. It is due to increase in size down the group.
Q.16. The stability of +5 oxidation state decreases and that of +3 state increases why?
Ans.Since energy required to unpair the two electrons of ns orbital is not compensated by energy released in formation of two extra bonds due to inert pair effect thus the stability of +5 oxidation state decreases and that of +3 state increases top to bottom.
Q.17.Give a example in which Bi has oxidation no 5.
Ans. BiF5
Q.18.What do you mean by disproportionation reaction explain with example?
Ans. In a particular reaction when same compound is reduced and oxidized the reaction is called disproportionation reaction.
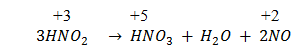
Q.19.When a particular compound of nitrogen goes under disproportionation reaction?
Ans.If oxidation no. of group 15 elements is, in its intermediate oxidation sate that is from from +1 to +4 that compound can go under disproportionation reaction.
Q.20.Nitrogen can show maximum covalency 4 but the heavier elements expand their covalence greater than 4 why?
Ans.Nitrogen has only four outermost orbitals that is one 2s and three 2p orbitals . Hence nitrogen can show maximum covalency 4 but heavier elements have vacant d orbitals hence they can expand their covalence greater than 4.
Q.21.Why phosphorus can forms PF–6 but nitrogen cannot?
Ans.Nitrogen can show maximum covalency 4 but the heavier elements can expand their covalence greater than 4.It is due to nitrogen has only four outermost orbitals that is one 2s and three 2p orbitals . Hence nitrogen can show maximum covalency 4 but heavier elements have vacant d orbitals hence they can expand their covalence greater than 4.
Q.22.Why nitrogen show anomalous behavior in its group?
Ans. It is due to
- Small size
- High electronegativity
- High ionisation enthalpy
- Non-availability of d orbitals in nitrogen atom.
Q.23.Nitrogen has unique ability to form ![]() multiple bonds with itself and with other elements why?
multiple bonds with itself and with other elements why?
Ans.It is due to small size and high electronegativity of nitrogen atom.
Q.24.Heavier elements of this group do not form ![]() bonds why?
bonds why?
Ans. Heavier elements d orbitals are so large and diffuse that they cannot have effective overlapping hence they do not form bond.
Q.25.Nitrogen exists as a diatomic molecule with a triple bond between the two atoms why?
Ans. Nitrogen has unique ability to form ![]() multiple bonds with itself due to small size and high electronegativity .
multiple bonds with itself due to small size and high electronegativity .
Q.26.Why is N2 inert at room temperature?
Ans. Nitrogen exists as a diatomic molecule with a triple bond between the two atoms and bond enthalpy of triple bond is very high.
Q.27.N–N bond is weaker than the single P–P bond why?
Ans.Due to small size of nitrogen atom inter electronic repulsion is developed between the two nitrogen atoms hence N–N bond can dissociate easily.
Q.28.Why catenation property of nitrogen is weaker than phosphorus?
Ans. N–N bond is weaker than the single P–P bond .It is due to small size of nitrogen atom inter electronic repulsion is developed between the two nitrogen atoms hence N–N bond can dissociate easily.
Q.29. Nitrogen cannot form ![]() bond but the heavier elements can e.g., R3P = O or R3P = CH2 (R = alkyl group.
bond but the heavier elements can e.g., R3P = O or R3P = CH2 (R = alkyl group.
Ans .It is due to non-availability of d orbitals in nitrogen atom.
Q.30.Arrange melting point of group 15 elements hydrides in decreases order.
Ans. NH3 > BiH3 > SbH3> AsH3 > PH3
From BiH3 To PH3 mass decreases hence intermolecular force of attraction decreases. NH3 has intermolecular H-bonding between their molecules hence boiling point is highest.
Q.31. Arrange boiling point of group 15 elements hydrides in decreasing order.
Ans. BiH3 > SbH3> NH3 > AsH3 > PH3
High boiling of NH3 is due to intermolecular H-bonding between their molecules
Q.32.Why boiling point of NH3 is higher than PH3?
Ans. High boiling of NH3 is due to intermolecular H-bonding between their molecules .In another case PH3 has no H-bonding between their molecules.
Q.33. Arrange bond length of E-H of group 15 elements hydrides in decreases order.
Ans. Bi-H( BiH3) > Sb-H (SbH3)> As-H (AsH3) > P-H (PH3) > N-H (NH3)
Because size of atom increases top to bottom.
Q.34.Arrange bond dissociation enthalpy of of E-H bond of group 15 elements hydrides in increasing order.
Ans. Bi-H( BiH3) < Sb-H (SbH3)<As-H <(AsH3) < P-H (PH3) < N-H (NH3)
Because bond length of E-H bond increases top to bottom.
Q.35. Arrange thermal stability of group 15 elements hydrides in decreasing order.
Ans. NH3 > PH3 > AsH3 > SbH3 > BiH3
Because bond dissociation enthalpy of of E-H bond of group 15 elements hydrides deceases top to bottom.
Q.36. Arrange reducing property of group 15 elements hydrides in increasing order.
Ans. NH3 <PH3 < AsH3 <SbH3 < BiH3
Because bond dissociation enthalpy of of E-H bond of group 15 elements deceases top to bottom.
Q.37.Why are group 15 elements hydrides Lewis bases.
Ans.This is due to presence of lone pair in group 15 elements hydrides.
Q.38. Arrange basicity of group 15 elements hydrides in increasing order.
Ans. NH3 > PH3 > AsH3 > SbH3 > BiH3
Ans.As we go down the group ability to attract H+ by hydrides decreases top to bottom. It is due to electron density at group 15 elements in hydrides decreases top to bottom.
Q.39.Arrange bond angle of H-E-H in group 15 elements hydrides in decreasing order.
Ans. NH3 > PH3 > AsH3 > SbH3 > BiH3.
Ans. All hydrides have pyramidal structure due to presence of one pair at central atom. As we go down the group size of the central atom increases hence bond pair –bond pair repulsion decreases hence bond angle decreases.
Q.40.Arrange basic properties of group 15 elements oxide in increasing order.
Ans. N2O3 –Acidic
P2O3-Acidic
As2O3-Acidic
Sb2O3-Amphoteric
Bi2O3-Basic.
Thus basic properties increases top to bottom.
Q.41.Which is more acidic in E2O3 and E2O5.
Ans. E2O5 is more acidic than E2O3.
The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state.
Q.42. Nitrogen does not form pentahalide why?
Ans. Nitrogen has only four outermost orbitals that is one s and three p orbitals and also it has no d orbitals,hence nitrogen cannot show maximum covalency greater than 4.
Q.43.Which is more covalent in PCl3 and PCl5.
Ans.PCl5 is more covalent than PCl3 because Pentahalides(oxidation no.of p is +5) are more covalent than trihalides(oxidation no.of p is +3).
Q.44.Which trihalide of nitrogen is stable.
Ans. NF3
Q.45.Which trihalide of group 15 elements are ionic in nature.
Ans. BiF3
Q.46. Mention the conditions required to maximise the formation of ammonia by Haber’s process.
Ans.Since Haber’s process is reversible and exothermic process and also no. of product molecules are lower than reactant molecules hence high pressure and low temperature would favor the formation of ammonia but temperature should not be very low otherwise reaction will be slow. Iron is used as a catalyst with molybdenum as a promoter.
Q.47.Ammonia gas is highly soluble in water why?
Ans.This is due to the ability of formation of H-bonding with water.
Q.48.Give examples of two neutral oxide of nitrogen.
Ans. N2O, NO.
Q.49.Give examples of acidic oxide of nitrogen.
Ans. N2O3, NO2, N2O4 ,N2O5
Q.50.Why does NO2 exist in dimer form?
Ans. NO2 contains a unpair electron, on dimerisation that is in N2O4 form its odd electron get paired hence NO2 exists in dimer form.
Q.51.What is the covalency of nitrogen in N2O5?
Ans.4.
Q.52. Some metals (e.g., Cr, Al) do not dissolve in concentrated nitric acid why?
Ans .This is due to the formation of a passive film of oxide on the surface of these metals.
Q.53.Describe the properties of important types of allotropes of phosphorus.
Ans.Important allotropes of phosphorus are red ,white and black.
| White phosphorus | Red phosphorus |
| 1.It exists as P4 molecule. 2.Poisonous. 3. Insoluble in water but soluble in carbon disulphide. 4.It glows in dark that is it show chemiluminescence or phosphorescence property. 5.It is more reactive than red phosporus. 6.Less stable | 1.It exists as polymeric molecule. 2. Non-poisonous. 3. Insoluble in water as well as in carbon disulphide. 4. It does not glow in dark. 5. It is less reactive than red phosphorus. 6.More stable |
Q.54.Draw the structure of white phosphorus and red phosphorus.
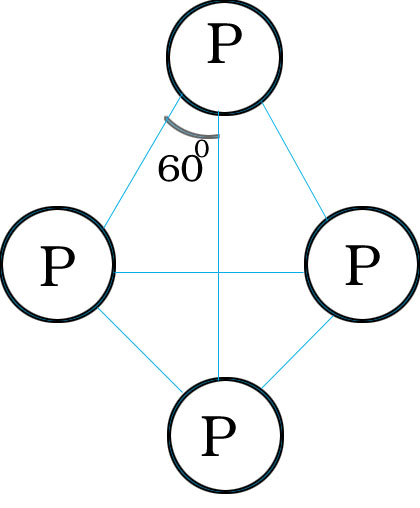
Ans.White phosphorus contains P4 molecules and all 4 atoms are tetrahedrally linked with each other.
Red phosphorus is polymer of white phosphorus.
Q.55.Why white phosphorus is more reactive than red phosphorus?
Ans. White phosphorous is more reactive than red phosphorous because white phosphorous has angular strain in P4 molecule where the angles are only 60o. There is no such angular strain in Red Phosphorous.
Q.56.Explain the types of black phosphorus.
Ans.There are two types of black phosphorus.
1. ![]() black phosphorus
black phosphorus
It is formed when red phosphorus is heated in a sealed tube at 803K
2. ![]() black phosphorus.
black phosphorus.
It is formed on heating white phosphorus at 473 K under high pressure
Q.5.Prove that PH3 is basic in nature.
Ans. Due to presence of lone pair, PH3 is basic in nature.
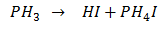
Q.57.In PH4+ and PH3 which has higher bond angle and Why?
Ans. Bond angle in PH4+ is higher than that in PH3. It is due to presence of lone pair on phosphorus in PH3 there is lp-bp repulsion so bond angle is lesser than the PH4+
Q.58.Are all the five bonds in PCl5 molecule equivalent explain?
Ans.

PCl5 has 5,P-Cl bonds in which three are equatorial bonds and two are axial bonds . The two axial bonds are longer than equatorial bonds. It is due to the axial bond pairs suffer more repulsion than equatorial bond pairs.
Q.59.In the solid state PCl5 exists as an ionic solid why?
Ans. In the solid state it exists as [PCl4]+ as cation and [PCl6]– as anion.
Q.60.H3PO4(Orthophosphoric acid) is tribasic why?
Ans.
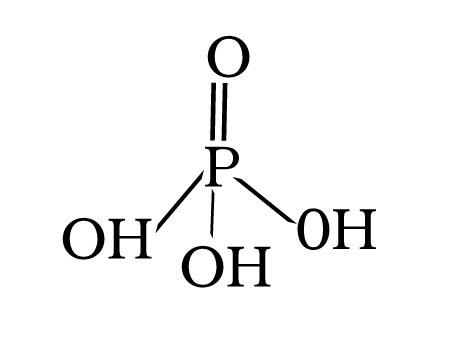
H3PO4 has three replaceable hydrogen because it contains three P-OH bonds hence it is tribasic.
Q.61.H3PO3(Orthophosphorous acid) is dibasic why?
Ans.
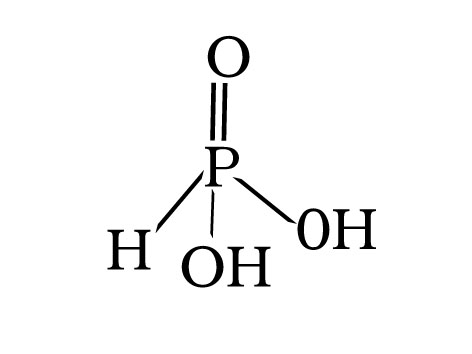
H3PO3 has two replaceable hydrogen because it contains two P-OH bonds hence it is dibasic.
Q.62.H3PO2(Hypophosphorous acid) is monobasic why?
Ans.

H3PO2 has only one replaceable hydrogen because it contains one P-OH bond hence it is monobasic.
Q.63.H3PO2 and H3PO3 show reducing property why?
Ans.Reducing property depends on the P-H bond since both molecules contain the hydrogen which is directly linked with phosphorus atom hence H3PO2 and H3PO3 show reducing property.
Group 16 elements.
O- Oxygen
S – Sulphur
Se-Selenium
Te-Tellurium
Po-Polonium
They are collectively called chalcogens.
Q.1.Which element is the most abundant in all the elements on earth?
Ans .Oxygen.
Q.2.What are the important source of the sulphur?
Ans. Eggs. proteins, garlic, onion, mustard, hair and wool etc.
Q.3.Why negative electron gain enthalpy of sulphur is higher than oxygen?
Ans.Due to very small size of oxygen atom electron density of oxygen is very high hence interelectronic repulsion is developed between oxygen atom and electron which to be added.
Q.4.Explain the metallic properties of group 16 elements.
Ans. O- Oxygen-Nonmetal
S – Sulphur- Nonmetal
Se-Selenium-Metalloids
Te-Tellurium-Metalloids
Po-Polonium-Metal
Hence metallic properties increases top to bottom,because size of the elements increases top to bottom and ionization enthalpy decreases top to bottom.
Q.5. The melting and boiling points of group 16 elements increase with an increase in atomic number down the group why?
Ans.Since mass of elements increases top to bottom hence vander waal force between their molecules increases hence the melting and boiling points of group 16 elements increase with an increase in atomic number down the group.
Q.6.There is large difference between the melting and boiling points of oxygen and sulphur why?
Ans.Sulphur exists in polyatomic state(S8) and oxygen exists in diatomic state(O2) hence difference between the melting and boiling points of oxygen and sulphur is high.
Q.7. Oxygen shows only negative oxidation state –2 why?
Ans.Due to very small size and high electronegativity of oxygen atom it shows only negative oxidation state –2 with some exceptions.
Q.8.In which compound oxygen show +2 oxidation state.
Ans. OF2
Q.10.The stability of + 6 oxidation state decreases down the group and stability of +4 oxidation state increases of group of 16 elements why?
Ans.As we go down the group energy required to unpair two ns electron is not compensated by energy release during formation of two extra bonds this effect is called inert pair effect and due to this inert pair effect stability of + 6 oxidation state decreases down the group and stability of +4 oxidation state increases of group of 16 elements
Q.11.Oxygen shows anomalous behavior in its group why?
Ans.It is due to
- Small size
- High electronegativity
- Non availability of d orbitals.
Q.12.Oxygen limits its covalency to four(theoretically) why?
Ans.Oxygen can show maximum covalency 4 but the heavier elements expand their covalence greater than 4.It is due to oxygen has only four outermost orbitals that is one s and three p orbitals . Hence oxygen can show maximum covalency 4 but heavier elements have vacant d orbitals hence they can expand their covalence greater than 4.
Q.13.Arrange melting and boiling point of group 16 elements hydrides in increasing order.
Ans.H2S< H2Se< H2Te<H2O.From H2S to H2Te molecular mass increase but presence of H-bonding in H2O molecules its melting point and boiling point is highest.
Q.14. Arrange E-H distance of group 16 elements hydrides in increasing order.
Ans. H-O( H2O )< H-S (H2S )< H-Se (H2Se)< H-Te(H2Te)
Because size of atom increases top to bottom.
Q.15.Arrange bond dissociation enthalpy of H–E bond of group 16 element hydrides in decreasing order.
Ans. H-O( H2O )> H-S (H2S)>H-Se (H2Se)> H-Te(H2Te)
Because size of atom increases top to bottom and thus H–E distance of group 16 elements hydrides increases top to bottom.
Q.16.Arrange thermal stability of group 16 elements hydrides in decreasing order.
Ans. H2O > H2S> H2Se> H2Te. Because bond dissociation enthalpy of H–E bond of group 16 elements hydrides decreases top to bottom.
Q17. Arrange bond angle of H-E-H of 16 element hydrides in decreasing order.
Ans. H2O > H2S> H2Se> H2Te.
All hydrides have bent structure due to presence of two lone pair electron at central atom. As we go down the group size of the central atom increases thus bond pair –bond pair repulsion decreases hence bond angle decreases.
Q.18.Arrange acidic property of group 16 element hydrides in decreasing order.
Ans. H2O < H2S< H2Se< H2Te.
As we down the group bond dissociation enthalpy of H–E bond of group 16 element hydrides decreases top to bottom.
Q.19.Why only hexafluoride is stable halide in hexahalide of group 16 elements?
Ans.It is due to small size and high electronegativity of fluorine atom bond strength of S-F bond is very high.
Q.20. Why SF6 is exceptionally stable.
Ans .Presence of six fluoride atoms produce high steric effect around sulphur atom.
Q.21 Arrange stability of halides of group 16 elements in decreasing order.
Ans. The stability of the halides decreases in the order F– > Cl– > Br– > I–.
Q.22.Give some examples of neutral oxide.
Ans. CO, NO and N2O.
Q.23. Give some examples of acidic oxide.
Ans. SO2, Cl2O7, CO2, N2O5
Q.24 Give some examples of basic oxide.
Ans. Na2O, CaO, BaO
Q.25 Give examples of amphoteric oxide.
Ans. Al2O3
Q.26.How is ozone layer useful for us?
Ans.Ozone layer protects the earth’s surface from dangerous ultraviolet (UV) radiations.
Q.27 Ozone is very unstable explain it thermodynamically.
Ans.
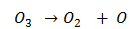
Since above reaction is an exothermic process hence ![]() is negative and also randomness increases thus
is negative and also randomness increases thus ![]() is positive. On combining these two values
is positive. On combining these two values ![]() of this reaction is large negative thus ozone is very unstable.
of this reaction is large negative thus ozone is very unstable.
Q.28.How is Ozone estimated quantitatively.
Ans. Step1: Ozone is reacted with an excess of potassium iodide solution buffered with a borate buffer iodine is liberated.
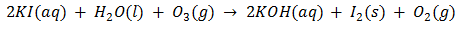
Step2: Liberated iodine is titrated against a standard solution of sodium thiosulphate, thus Ozone is estimated quantitatively.
Q.29.Why ozone is powerful oxidizing agent?
Ans.![]()
Due to formation of nascent oxygen ozone is powerful oxidizing agent.
Q.30.How does jet aeroplanes deplete the ozone layer.
Ans .Exhaust gas reacts with ozone layer.

Q.31.Draw the resonating structure of O3. Are the two O-O bonds identical?
Ans.
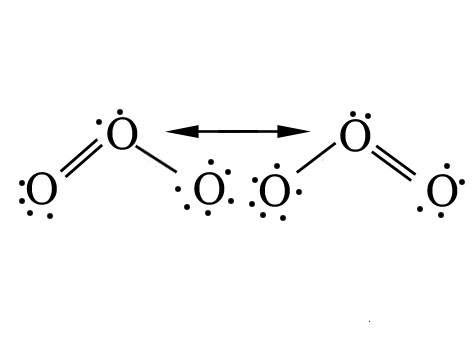
The two O-O bond lengths in the ozone molecule are identical due to resonance.
Q.32.Explain the properties of different types of allotropes of sulphur.
Ans.Sulphur has many allotropic forms but the two allotropes are very important .
1.Rhombic sulphur
2.Monoclinic sulphur
| Rhombic sulphur(alpha sulphur) | Monoclinic sulphur (beta sulphur) |
| Stable form at room temperature | When Rhombic sulphur is heated above 369 K monoclinic sulphur is obtained. |
| It is insoluble in water but soluble in CS2 | It is soluble in CS2 |
| Contains S8 molecules | Contains S8 molecules |
Q.33.Why is S2 paramagnetic in nature.
Ans.Accroding to molecular orbital theory S2 has two unpair electron in antibonding ![]() orbitals hence S2 is paramagnetic in nature.
orbitals hence S2 is paramagnetic in nature.
Q.34.Draw the structures of rhombic sulphur in S8 ring and S6 form.
Ans.
In S8 form (Rhombic sulphur)

In S6 form.

Q.35. Draw the resonating structure of SO2. Are the two S-O bonds identical?
Ans.
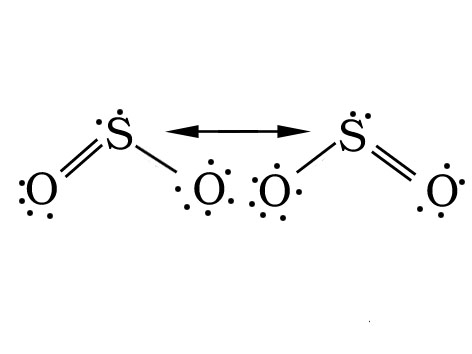
The two S-O bond lengths in the SO2 molecule are identical due to resonance.
Q.36.Which oxoacids of sulphur has peroxy linkage.
Ans. H2S2O8
Q.37.What are the conditions to maximise the formation of H2SO4 by contact process.
Ans.
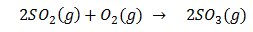
In Contact process SO2 is first is oxidized to SO3. Since this reaction is exothermic, reversible reaction and also number of gaseous molecules of product is smaller than reactant.Hence low temperature and high pressureare the favourable conditions according to Le-Chtalier principle but the temperature should not be very low otherwise rate of reaction will become slow. V2O5 is used as catalyst.
Q.38.Explain the properties of H2SO4 which decides the reactivity of it.
Ans. (a) Less volatile.
(b) Strong acid.
(c) strong affinity for water.
(d) Strong oxidising agent
Q.39.Why is Ka2 << Ka1 for H2SO4 in water?
Ans. H2SO4 is acidic in nature loss H+ in two steps but it largely dissociates in first step.
Step 1-

Step2-
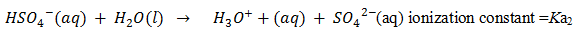
that is why Ka2 << Ka1 for H2 SO4 in water
Q.40.List some important use of H2SO4.
Ans.1.Industrial applications
a.Manufacture of fertilizers
b.Manufactuing paints, pigments etc.
2.Metallurgical applications.
Cleansing metals before enameling, electroplating and galvanizing.
3.In lead storage batteries.
Group 17 elements(Halogens)
F- Fluorine
Cl – Chlorine
Br – Bromine
I – Iodine
At- Astatine(Radioactive)
Q.1 Why are halogens obtained mainly from sea water specially chlorine?
Ans.Sea water contains mainly sodium chloride solution but it also contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium.
Q.2.Group 17 elements have very high ionisation enthalpy why?
Ans.Due to their smallest size in respective period and presence of 7 electrons in their outermost orbital they have little tendency to lose electron. Group 17 elements have very high ionisation enthalpy.
Q.3. Halogens have maximum negative electron gain enthalpy in the respective periods of the periodic table why?
Ans.Halogens are smallest in their respective periods thus having high effective nuclear charge thus they can accept electron easily.
Q.4.What are the physical states of halogens?
Ans. F2– Fluorine-Gas
Cl2– Chlorine-Gas
Br2 – Bromine -Liquid
I2 – Iodine-Solid
Q.5.Arrange melting and boiling point of halogens in increasing order.
Ans. F2 <Cl2< Br2 <I2
As mass of the halogen increases intermolecular Vander waal force of attraction increases thus melting and boiling point increases.
Q.6.Why is –ve electron gain enthalpy of chlorine higher than Fluorine?
Ans.Due to very small size of fluorine atom electron density on fluorine atom is very high,thus inter electronic repulsion is developed between fluorine atom and electron which to be added.Thus –ve electron gain enthalpy of chlorine is higher than Fluorine.
Q.7.Arrange electronegativity of halogens in decreasing order.
Ans.F >Cl> Br> I.Since size decreases top to bottom hence ability to gain electron decreases top to bottom.
Q.8.Halogens are coloured why?
Ans.Halogens absorb light in visible region and thus valence electrons get excited to higher energy level and thus show colour.
Q.9.Arrange bond dissociation enthalpy of halogens in decreasing order.
Ans. Cl2>Br2> F2 >I2
Q.10.Enthalpy of dissociation of F2 is smaller than Cl2 why?
Ans.Fluorine atom is smaller in size thus interelectronic repulsion is developed between lone pairs of two fluorine atoms thus dissociation of this bond become easier.
Q.11.Arrange oxidizing property of halogens in decreasing order.
Ans. Since –ve electron gain enthalpy of halogen atom and hydration enthalpy of halogen ion decreases top to bottom hence standard reduction potential value (Eo) value of halogens decreases from fluorine to Iodine thus oxidizing property of halogens decreases from top to bottom in group.
F2 >Cl2>Br2 >I2
Q.12.-Ve electron gain enthalpy of chlorine is higher than fluorine atom yet fluorine is stronger oxidizing than chlorine.
Ans.The two factors which make oxidising power of fluorine more than that of chlorine are as follows.
1.The lower bond dissociation enthalpy of F-F bond than Cl-Cl.
2.Higher hydration enthalpy of F– than Cl–
Q.13.Fluorine shows only one -1 oxidation state and cannot expand its octet why?
Ans.Fluorine is most electronegative atom and has no d orbitals hence fluorine show only one -1 oxidation state.
Q.14. Other halogens exhibit + 1, + 3, + 5 and + 7 oxidation states but fluorine show only one -1 oxidation state and cannot expand its octet why?
Ans.Fluorine is most electronegative atom and has no d orbital hence fluorine show only one -1 oxidation state. Other halogens have d orbitals and therefore, can expand their octets and show + 1, + 3, + 5 and + 7 oxidation states also.
Q.15. Halogens are highly reactive why?
Ans.Due to their small size and high electronegativity halogens can react with metals and nonmetals and even among themselves, hence halogens are highly reactive.
Q16. Is following reaction feasible?
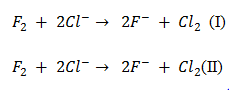
Ans.
In above reactions II is feasible because fluorine is reduced and chlorine is oxidized since fluorine is stronger oxidizing agent than chlorine
Q.17. Why fluorine shows anomalous behaviour in its group.
Ans.It is due to following reasons.
1.Small size
2.High ionization enthalpy
3.High electronegativity
4. Non availability of d orbitals
Q.18.List some anomalous behaviour of fluorine.
Ans.1. It forms only one oxoacid(HOF) while other halogens form a number of oxoacids.
2.Hydrogen fluoride is liquid but other halides are gases.
3.Bond dissociation enthalpy of F-F is lower than Cl-Cl
4. It exhibits only –1 oxidation state
5. Most of the reactions of fluorine are exothermic .
Q.19.Arrange melting and boiling point of group 17 elements hydrogen halides in increasing order.
Ans. HCl < HBr < HF < HI (melting point)
HCl < HBr < HI< HF(Boiling point)
Q.20.Why boiling point of HF is highest among group 17 elements hydrogen halides?
Ans.Due to presence of H-bonding between HF molecules boiling point of HF is highest.
Q.21.Arrange bond length of H-X of group 17 elements hydrogen halides in incresing order.
Ans. H-F< H-Cl < H-Br < H-I
Because size of the halogen increases top to bottom.
Q.22.Arrange bond dissociation enthalpy of group 17 elements hydrogen halides(H-X) in decresing order.
Ans. H-F> H-Cl >H-Br > H-I
Above order is due to bond length of group 17 elements hydrogen halide (H-X) increases top to bottom.
Q.23.Arrange thermal stability of group 17 elements hydrogen halides(H-X) in decreasing order.
Ans. H-F> H-Cl >H-Br > H-I
Above order is due to bond dissociation enthalpy of group 17 elements hydrogen halides (H-X) decreases top to bottom.
Q.24.Arrange acidic property of group 17 elements hydrogen halides(H-X) in increasing order.
Ans.H-F< H-Cl < H-Br < H-I
Above order is due to bond dissociation enthalpy of group 17 elements hydrogen halides (H-X) decreases top to bottom
Q.25.Why is OF2 called oxygen fluoride?
Ans.It is due to higher electronegativity of fluorine than oxygen.
Q.26.What are the use of ClO2?
Ans.ClO2 is used as a bleaching agent for paper pulp and textiles.
Q.27.Arrange Ionic character of metal halides (MF ,MCl , MBr , MI) in decreasing order.
Ans. MF >MCl > MBr > MI(M is monovalent metal)
As the size of halogen increases its polarizing power increases hence covalent character increases and thus ionic character decreases.
Q.28.Which is more covalent in SnCl2 and SnCl4
Ans.SnCl4 is more covalent than SnCl2
It is due to higher oxidation state of Sn(+4) in SnCl4 than SnCl2
Q.29.What are interhalogens?
Ans.Halogens react amongst themselves and form a number of compounds called Interhalogens.
Types of interhalogens.
XX’3
XX’5
XX’7
where X is a larger size halogen and is smaller size halogen
Q.30.Why is Cl2 powerful bleaching agent?
Ans.Due to formation of nascent oxygen Cl2 is powerful bleaching agent.The bleaching action of Chlorine is permanent because it involves the process of oxidation.
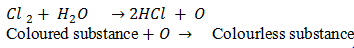
Q.31.Examples of three poisonous gases which can be formed from chlorine gas.
Ans.
- Phosgene gas (COCl2)
- Tear gas(CCl3NO2)
- Mustard gas (ClCH2CH2SCH2CH2Cl)
Q.32.What is aqua regia.
Ans.Mixture of three parts of concentrated HCl and one part of concentrated HNO3 is called aqua regia.It is used for dissolving noble metals such as gold, platinum etc.
Q.33.Arrange acidic and oxidising property of HOCl, HOCIO , HOCIO2, HOCIO3 In increasing order.
Ans. HOCl < HOCIO < HOCIO2 <HOCIO3 (Acidic Strength)
Oxygen is more electronegative than chlorine. With an increase in the number of O atoms attached to Cl, more electrons are pulled away from O−H bond and more weaker becomes the O−H bond. This increases the acid strength.
HOCl > HOCIO > HOCIO2 > HOCIO3 (Oxidising Power)
because the oxidizing power of oxyacids of chlorine is inversely related to the thermal stability of these acids i.e. higher the thermal stability, lower will be oxidizing power of the oxyacid and vice versa. For example the HClO4 is most stable so it has lowest oxidizing power amongst the oxyacids of chlorine.
Q.34.Among interhalogens, IF7 have maximum number of halogen atoms why?
Ans.It is due to the ratio of radii between I and F is maximum.
Q.35.Interhalogen compounds are more reactive than halogens (except fluorine) why?
Ans. This is due to X-X’ bond in interhalogens is weaker than X–X bond in halogens except F–F bond.
Q.36.Explain the properties of interhalogens.
Ans.1. Covalent in nature.
2. Diamagnetic
3. Physical properties are intermediate between those of constituent halogens.
4. More reactive than halogens except fluorine.
Group 18 elements (Noble gases)
He- Helium
Ne- Neon
Ar- Argon
Kr- Krypton
Xe- Xenon
Rn- Radon
Q.1.Why are group 18 elements called noble gases?
Ans. Group 18 elements have their completely filled valence shell orbitals hence react with a few elements thus they are now known as noble gases.
Q.2.Why group 18 elements have very high ionisation enthalpy?
Ans. Group 18 elements have their completely filled valence shell orbitals hence they have very little tendency to loss electron thus they have very high ionisation enthalpy.
Q.3. Why Group 18 elements have +ve electron gain enthalpy.
Ans. Group 18 elements have their completely filled valence shell orbitals hence they have no tendency gain electron thus they have +ve electron gain enthalpy.
Q.4.Boiling points of noble gases are very low why?
Ans.Noble gases atoms are joined with each other by weak dispersion force hence they can liquefy easily.
Q.5.Why only Xe forms some compound with most electronegative fluorine and oxygen atom?
Ans.Due to its large size and and low ionization enthalpy it can forms compound with most electronegative fluorine and oxygen atom.
Q.6.Why the chemistry of radon is difficult?
Ans.Due to its radioactive nature with very short half life period.
Q.7.What are the uses of He?
Ans.1.Due to its low solubility in blood it is used as diluents for oxygen in diving apparatus.
2. In filling balloons for meteorological observations.
3. Liquid helium used as cryogenic agent for carrying out various experiments at low temperatures.
4. In gas-cooled nuclear reactors.
Q.8.What are the uses of Ne?
Ans.1.In discharge tubes and fluorescent bulbs for advertisement display.
2. Neon bulbs are used in botanical gardens and in green houses
Q.9.What are the uses of Ar?
Ans.1.To provide an inert atmosphere in high temperature metallurgical processes.
2.For filling electric bulbs.
Structures.
Draw structures of
1.ClF3
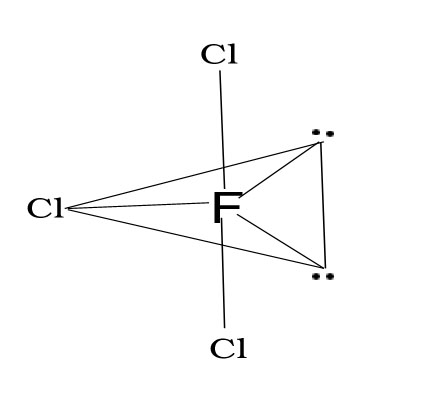
T –shape(Slightly bent)
2.SF4

See-saw shape.
3.XeF2
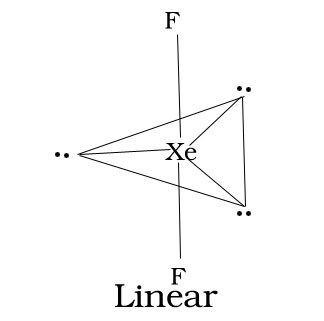
4.XeF4

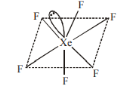
5.XeF6
Distorted Octahedral
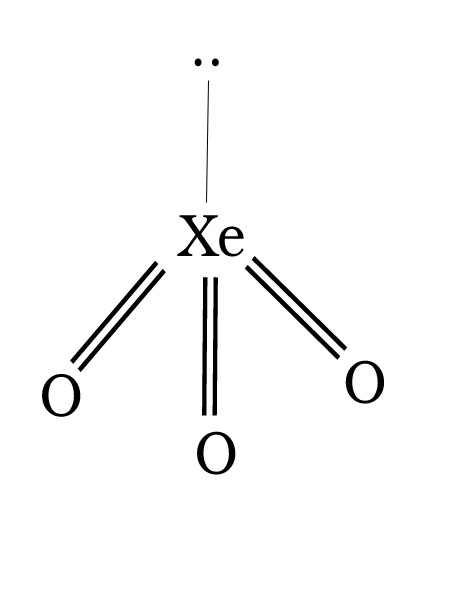
6. XeO3
Pyramidal