Organic Chemistry-Some basic Principles and techniques
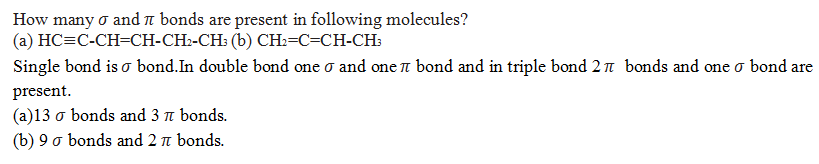
What is the hybridization state of each carbon in the following compounds?
(a) CH3-CH2Cl, (b) C6H6, (c) CH2=C=CH-CH3
Methods for determination of hybridization state of carbon.
| No. of atoms attached to carbon | Hybridisation state. |
| 2 | Sp |
| 3 | Sp2 |
| 4 | Sp3 |
(a) Sp3, Sp3
(b) All carbon are Sp2 hybridised.
(c) Sp2 ,Sp ,Sp2, Sp3
Represent 2-methylbutane in condensed form ,complete structural form and bond line form.
Condensed form-CH3CH(CH3)CH2CH3
Complete structural form
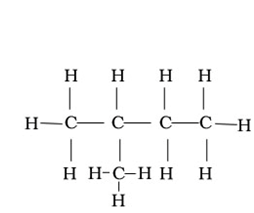
Bond line form
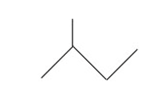
What are the common names of following Alicyclic compounds?
Alkane family
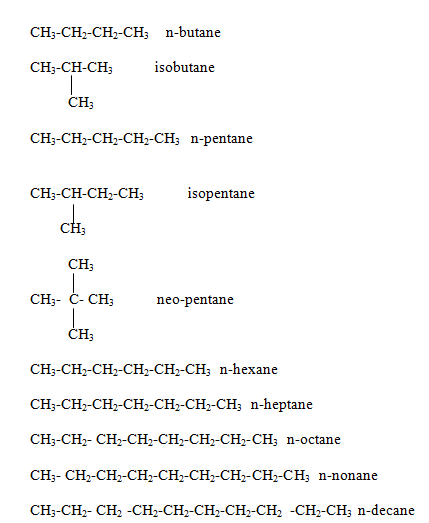
Alkene family
CH2=CH2 Ethylene
Alkyne family
Name of alkyl Groups.

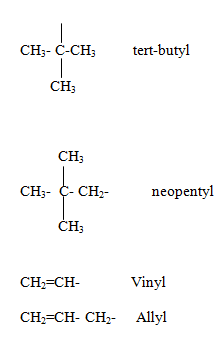
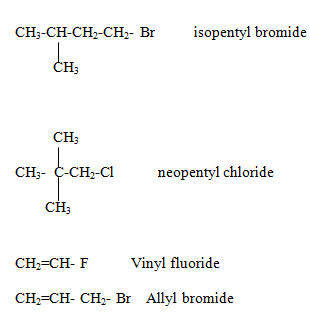
Naming of alkyl halide family
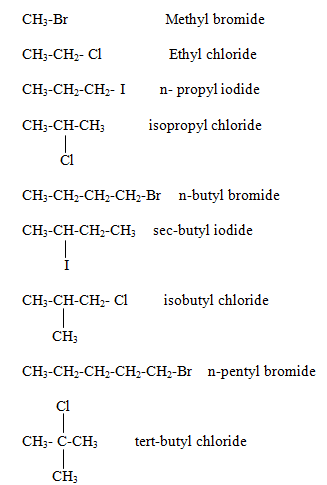
Naming of Alcohol family
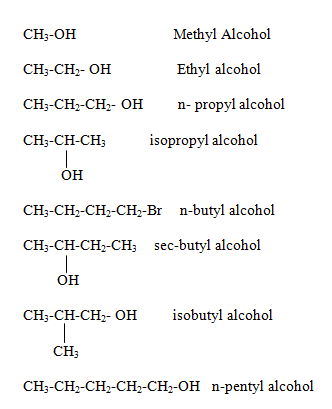
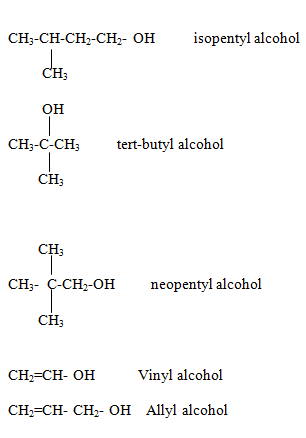
Aldehyde family
H-CHO Formaldehyde
CH3-CHO Acetaldehyde
Ketone family
CH3-CO-CH3 Acetone
Carboxylic acid
H-COOH Formic acid
CH3-COOH Acetic acid
Ester family
H-COOCH3 Methyl formate
CH3-COOCH2-CH3 Ethyl acetate
Acid Amide family
H-CONH2 Formamide
CH3-CONH2 Acetamide
Acid halide family
H-COCl Formyl chloride
CH3-COBr Acetyl bromide
Acid anhydride family
(CH3-CO)2O Acetic anhydride
Ether family
CH3-O-CH3 dimethyl ether
C2H5-O-C2H5 diethyl ether
(CH3)3C-O-CH2-CH3 tert-butyl ethyl ether
Amines family
Primary amine(1oAmine)
CH3-NH2 Methyl amine
CH3-CH2– NH2 Ethyl amine
CH3-CH2-CH2– NH2 n- propyl amine
Secondary amine(2oAmine)
CH3-NH2 Methyl amine
CH3-CH2– NH- CH2-CH3 diethyl amine
CH3-CH2-CH2– NH- CH2-CH3 Ethyl n- propyl amine
Tertiary amine(3oAmine)
(CH3)3-N trimethyl amine
Rules for IUPAC naming of organic compounds.
Alkane family
(i)Select the longest chain 1st
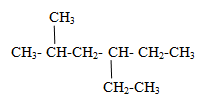
(ii) Give the parent name.
Since the longest chain containing 6 carbons hence parent name is hexane.
(iii) If more than one longest chains are present select that chain which follow lowest locant rule
Lowest locant rule :The lowest set of locants is defined as the set that, when compared term by term with other locant sets, each cited in order of increasing value, has the lowest term at the first point of difference; for example, the locant set ‘2,3,5,8’ is lower than ‘3,4,6,8’ and ‘2,4,5,7’
In above example if we go from left side branches come at 2 and 4 and from right side 3 and 5. According to Lowest locant rule we will go from left.
(iv) If the two branches are present at equivalent positions, take that chain which give
lower position of that branch which come first in alphabetical order.
(v) While giving the name of an alkyl group ,that carbon atom of branch is numbered 1
which attaches to the main chain of alkane
(vi) Write all branches before parent name in alphabetical order.
Thus mane of above compound is 4-ethyl-2-methylhexane.
(vi) The prefixes iso- and neo- are considered to be the part of an alkyl group,and the
prefixes sec– and tert– are not considered to be the part of the alkyl group.
(vii) If two chains having same length then that chain is taken which contains more number of branches.
(viii) Use di (for 2), tri (for 3), tetra (for 4), penta (for 5), hexa (for 6) if substituents come more than one times.
Alkene family
Rule (i)The longest chain is selected containing double bond and give the parent name as alk-(position of double bond)-ene. Priority is given to double bond so that it gets lowest position.
Example:

Rule (ii)Others rule are similar with alkane.
Thus above compound is 3-ethyl-5-methylhex-2-ene.
Name of polyalkene.
Rule (i)The longest chain is selected containing maximum double bonds and give the parent name as alka-(positions of double bonds)-polyene (where poly is di,tri,tetra etc depending on mumber of double bonds). Longest chain is selected such a way that it obeys lowest sum rule.
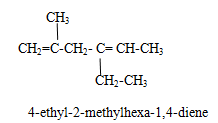
Alkyne family
Rule (i)The longest chain is selected containing triple bond and give the parent name as alk-position of triple bond-yne. Priority is given to triple bond so that it gets lowest position.

Hex-1-yne
Rule (ii) Others rule are similar as alkane. Thus above compound is 3-ethyl-5-methylhex-1-yne
Name of polyalkyne family.
All rules are similar with polyalkene but -ene is replaced with -yne
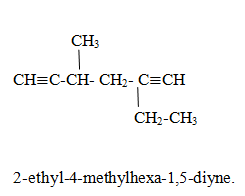
Compounds containing both double bonds and triple bonds.
Rule(i) Priority is given to double bond so that it gets lowest position.
Parent name is Alk-position of double bond-en-position of triple bond-yne.
Rule1. Use lowest Locant Rule. If lowest locant Rule is same give priority to the double bond.
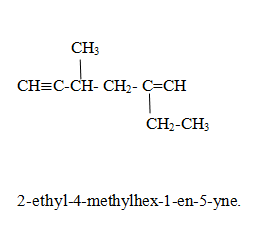
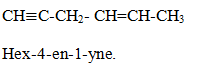
Naming of alkyl halide family
Halogens are considered as substituent only and write them with other substituent in alphabetical order.
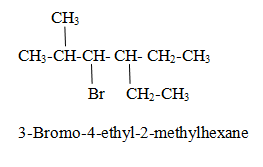
Alcohol family
General formula is R-OH
Where R is an alkyl group.
Rule (i) The longest chain is selected containing –OH group and give the parent name as alkan-position of alcohol-ol. Priority is given to –OH group so that it gets lowest position.
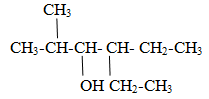
Naming of alcohol containing multiple –OH groups.
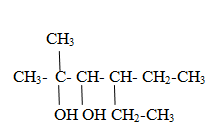
Rule-The longest chain is selected containing maximum –OH groups and give the parent name as alkane-(positions of –OH groups)-polyol (where poly is di,tri,tetra etc depending on mumber of OH groups). Longest chain is selected such a way that it obeys lowest locant rule.
Thus name of above compound is 4-ethyl-2-methyl hexane-2.3-diol.
Compounds containing alcohol and double bond
The longest chain is selected containing both double bond and alcohol group and give the parent name as alk-position of double bond-en-position of –OH group-ol Priority is given to –OH group so that it gets lowest position.

Compounds containing alcohol and triple bond
The longest chain is selected containing both triple bond alcohol group and give the parent name as alk-position of triple bond-yn-position of –OH group-ol Priority is given to –OH group so that it gets lowest position.
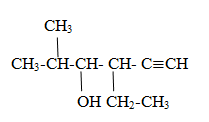
Naming of aldehyde family
Rule(i)The carbon of functional group is numbered 1 and parent name is given as alkanal.
(ii)All others rule are similar with alkane

Compounds containing multiple –CHO group.
Case1 When compounds contain two –CHO group.
Rule: One –CHO is numbered 1 and another is last.
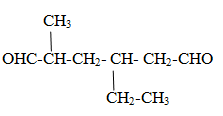
Parent name is given as alkane-positions of aldehydic groups-dial
4-ethyl-2-methylhexane-1,6-dial
CaseII When compounds contain more than two –CHO groups and all –CHO groups are attached in main chain.

Case III When compounds contain more than two –CHO groups and one of the–CHO group is not attached in main chain.

Compounds containing double bond/triple bond and aldehydic group.
All rules are similar when alcohol contain double bond/triple bond but –ol is replaced by al.
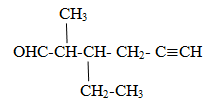
Naming of ketone family

Rule:The longest chain is selected containing ketonic group and give the parent name as alkan-position of ketonic group -one Priority is given -CO group so that it gets lowest position.
Rule (ii) Others rule are similar with alkane.
Thus above compound is 4-ethyl-2-methylhexan-3-one
Compounds containing polyketonic geoup.
Rule-The longest chain is selected containing maximum –CO groups and give the parent name as alkane-positions of –CO groups-polyone(where poly is di,tri,tetra etc depending on number of CO groups). Longest chain is selected such a way that it obeys lowest locant rule.

Thus name of above compound is 3-ethyl-5-methylhexane-2,4-dione
Compounds containing double bond/triple bond and ketonic group.
All rules are similar when alcohol contain double bond/triple bond but –ol is replaced by one.

Naming of carboxylic acid.
Rule(i)The carbon of functional group is numbered 1 and parent name is given as alkanoic acid.
(ii)All others rule are similar with alkane

Compounds containing multiple –COOH group.
Case1 When compounds contain two –COOH group.
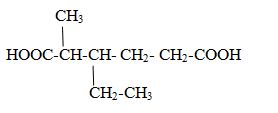
Rule:One –COOH is numbered 1 and another is last.
Parent name is given as alkane-positions of carboxylic group-dioic acid
3-ethyl-2-methylhexane-1,6-dioic acid.
CaseII When compounds contain more than two –COOH groups and all –COOH groups are attached in main chain.
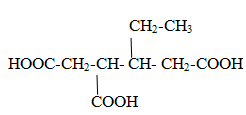
Case III When compounds contain more than two –COOH groups and one of the–COOH group is not attached in main chain.

Compounds containing double bond/triple bond and acidic group

Naming of Acid halide
Functional group is –COX(where X is halogen)
The carbon of functional group is numbered 1 and parent name is given as alkanoyl halide.
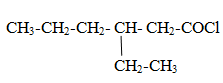
Naming of Acid amide
Functional group is –CONH2
The carbon of functional group is numbered 1 and parent name is given as alkanamide
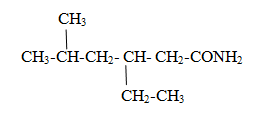
Naming of Acid ester
Functional group is –COOR
The carbon of functional group is numbered 1 and parent name is given as alkanoate.
Alkyl group with ester group written as separate word.
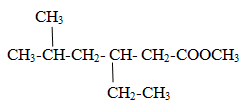
Naming of Acid anhydride

Naming of ether family.
General formulas- R-O-R
CH3-CH2-O-CH3
Smaller O-R group is considered as branch and written as alkoxy .
Hence name of above compound is methoxyethane.
Name of nitrile family
General formula R-CN
The carbon of functional group is numbered 1 and parent name is given as alkanenitrile.
CH3-CH2-CH2-CH2-CN
Hence name of above compound is Pentanenitrile.
Multiple -CN group
CN-CH2-CH2-CH2-CN
Pentane-1,5-dinitrile.

Naming of amine family.
Primary amine
General formula R-NH2
CH3-CH2-CH2-CH2-NH2
Longest chain is selected containing –NH2 group and parent name is
alkan-position of NH2-amine
hence name is above compound is butan-1-amine
Secondary amine
General formula R-NH-R
CH3-CH2-CH2-CH2-NH- CH2-CH3
Rule (i) Longest chain is selected containing –NH on either side of N and parent name is
alkan-position of NH-amine
Rule(ii)Alkyl on other side of –NH is written as branch before parent name.
hence name is above compound is N-ethylbutan-1-amine
Tertiary amine
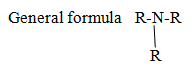

Naming of aliphatic compound containing multiple functional groups.
In given multiple groups family is decided by priority rule. Other groups are written as branch.
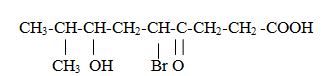
Priority order :
-COOH(Carboxyl grpoup), –SO3H(sulphonic), (RCO)2O(anhydrides)-COOR(esters ) (R=alkyl group), COCl(acid chlorides),-CONH2((acid amides), -CN(nitriles),-HC=O(aldehydes), >C=O(ketones), -OH(alcohol), -NH2(amines),, >C=C(alkenes)<,-C≡ C-
–R, C6H5-, halogens (F, Cl, Br, I), –NO2, alkoxy (–OR) etc. are always considered as substituent.
Prefix names of branches when they are considered as branches.
| groups | Prefix name |
| -COOH -COOR -COX -CONH2 -SO3H -CN -HC=O >C=O -OH -NH2 | carboxy alkoxycarbonyl halocarbonyl carbamoyl sulpho cyano Formyl or oxo oxo hydroxyl amino |
Naming of cyclic aliphatic(alicyclic) compound.
Category I
Category II
In this category side chain contains higher number of carbon atoms than in ring. Side chain is considered as parent chain.

In this category side contain lower number of carbon atoms than in ring. Side chains are written as substituent according to lowest sum rule. If only two side chains are present give position one to that which comes alphabetically first.
Category III
In this category side contains lower number of carbon atoms than in ring. Side chains are written as substituent according to lowest locant rule. If only two side chains are present give position one to that which comes alphabetically first.
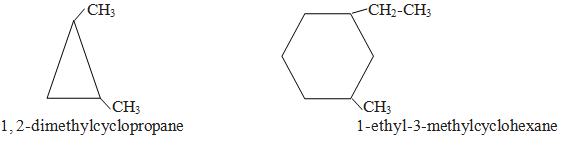
Category IV
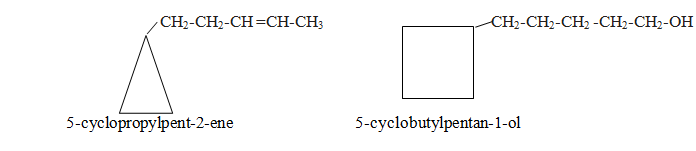
In this category side chain contain either double or triple bond or functional groups.Side chain is written as main chain and ring becomes substituent.
Category V
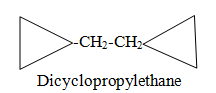
When more than one rings are present in single chain.
Category VI
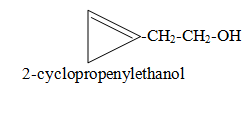
When side chain contains functional group and ring contains double or triple bond. Side chain is considered as main chain.
Category VII

When side chain as well as ring contain same functional group. Which will be considered as main chain it depends on number of carbon atoms.
Category VIII
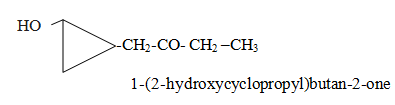
When side chain as well as ring contain different functional group.Which will be considered as main chain it depends on the priority order.
Category IX
When a compound contains benzene ring and and alicyclic ring in same chain.
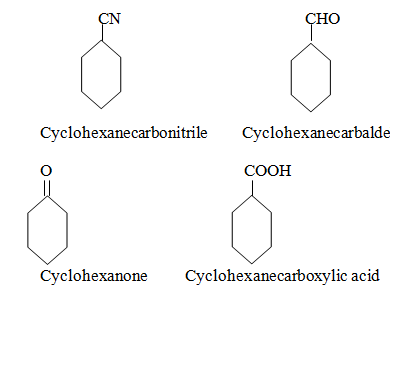
Name of some other compounds.
Naming of aromatic compound.
Category I
 |  |  |
 |  |  |
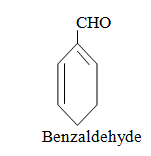 | 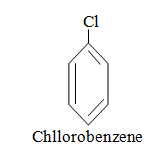 |  |
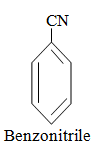 | 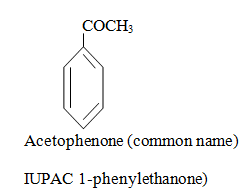 | 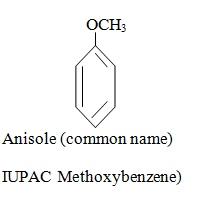 |
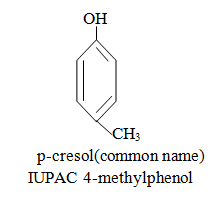 | 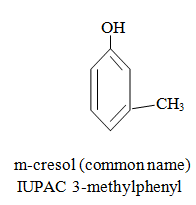 | 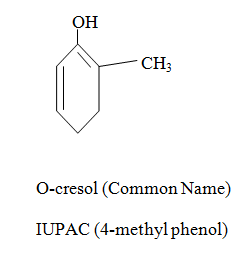 |
When aromatic compound contains substituents only.

Parent name is benzene and write all branches before parent name with their positions according to lowest locant rule. If lowest locant rule is same, group which come alphabetically first should be at lowest position.
When benzene doesn’t contain principal functional group groups.
When aromatic compound contains more than one function groups.

When aromatic compound contains more than one functional groups of same type.

Isomers
Compounds having same molecular formula but different structural formula called isomers.
Type of isomers:
Structural isomers-Isomers having different bonding pattern that is different positions of double bonds/functional groups/substituents are called structural isomers.
Stereo isomers-Isomers having same bonding pattern but differ in relative positions of their atoms or groups in space are called stereoisomers.
Position isomers-
In position isomerism, the basic carbon skeleton remains unchanged, but important groups are moved around on that skeleton.

Chain isomers-
Isomers having difference in main chain are called chain isomers. Chain isomers are molecules with the same molecular formula, but different arrangements of the carbon ‘skeleton’
Example:

Functional isomers-
Isomers having difference in functional groups are called functional isomers.
Example:
CH3-CH2-CH2-CH2 OH CH3-CH2-O-CH2-CH3
Metamers-
Isomers having difference in alkyl chains on either side of the functional group
in the molecule are called metamers . Example:
CH3-CH2-CH2-CH2 OH CH3-CH2-O-CH2-CH3
Division of the covalent bond
There are two types of division of covalent bond.
(i) Homolytic division
(ii) Heterolytic division
(i) Homolytic division-Type of division in which one electron of the shared pair in a covalent bond goes with each of the combining atoms. Free radicals are formed during this division.
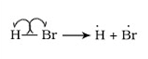
(ii) Heterolytic division-Type of division in which the shared pair of electrons remains with one of the combining atom.Cations and anions are formed during this division.

Q.Define carbocation(carbonium ion) and carboanions.
Ans.Species having a carbon atom containing +ve charge and 6 electrons called carbocation.
Example: CH3CH2+
Species having a carbon atom containing -ve charge and 8 electrons called anions.
Example:
Types of carbocation:
There are three types of carbocation.
Primary carbocation(1o)-CH3CH2+
Seconary carbocation(2o) CH3-CH+-CH3
Tertiary carbocation(3o)-(CH3)3C+
What is free radicals.
Species which contains unpaired electron called free radicals.
Define different types of chemical reagent.There are two types of reagent
(i)Electrophile
Electrophile is the molecule or ion that is electron deficient and accept a pair of electrons to make a new covalent bond.
Examples:
(i) Cations are electrophile like CH3+, CH3CH2+ etc
(ii) Electron deficient compounds are electrophile AlCl3,BF3
(ii) Nucleophile:
Atoms or molecules which can donate electron are called nucleophile
Examples:
(i) Anions are electrophile like Cl–,OH–, CN– etc
(ii) Electron deficient compounds are electrophile like H2O,NH3
Explain inductive (I) effect.
Inductive effect is an effect in which permanent polarization arises due to partial displacement of sigma e- along carbon chain or partial displacement of sigma-bonded electron toward more electronegative atom in carbon chain i.e.
There are two types of inductive effect
(i)+I effect.
When an atom or group donates electrons towards the bond and acquires a partial positive charge, the atom or group is called +I group, and the corresponding effect is called the positive Inductive Effect or the +I effect.
Example of +I groups: – O –, – COO –, –CR3, –CHR2, –CH2R, –CH3, –D
(ii)-I effect
When an atom or group attracts the bonding electrons towards itself and acquires a partial negative charge, the atom or group is called -I group and the corresponding effect is called the electron-withdrawing inductive effect, or the -I effect.
Example of -I groups: –NO2, – SO2R, –CN, –SO2Ar, –COOH, –F, – Cl, – Br, – I, –OAr, –COOR, –OR, –COR, –SH, –SR, –OH, –Ar, – CH = CR2
Inductive Effect Order
The following groups are listed approximately in order of decreasing strength for both – I and + I effects.
Inductive Effect Order for +I Groups
– O –> – COO –> –CR3 > –CHR2 > –CH2R > –CH3 > –D
Inductive Effect Order for -I Groups
-NR3+ > -SR2+ > -NH3+ > –NO2 > – SO2R > –CN > –SO2Ar > –COOH > –F > – Cl > – Br > – I > –OAr > –COOR > –OR > –COR > –SH > –SR > –OH > –Ar > – CH = CR2
Q.What are the applications of inductive effect?
Ans.(i)To determine stability of compound.
Example: Which is more stable in CH3-CH2-CH2– and NO2-CH2-CH2–
NO2-CH2-CH2–is more stable than CH3-CH2-CH2–. -I effect of NO2 disperse the –ve charge whereas +I effect CH3-CH2-CH2 of does not.
Arrange the stability of primary,secondary ,tertiary carbocation in increasing order.

As the number of pushing alkyl groups increases dispersion of +ve charge increases thus stability increases.
Arrange the stability of primary,secondary ,tertiary carboanion in decreasing order.

As the number of pushing alkyl groups increases the density of -ve charge increases thus stability decreases.
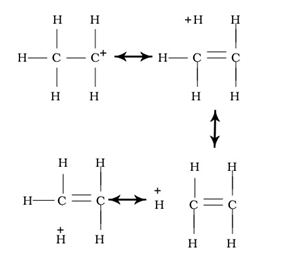
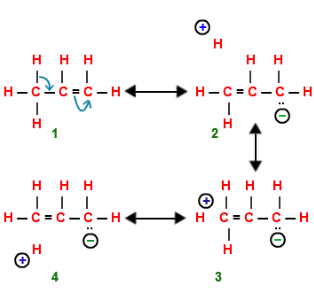
Define resonance.
When a molecule is represented by two or more structures and that structures are different in the position of electrons not in position of atoms, then the structure is called as resonating structure and this phenomenon is called as resonance..
Condition for resonance effect.
Resonance is possible in only in following conditions.
(i) Molecule should be planer, nearly planer or a part of it is planer
(ii) Molecule should posses conjugated system.
Conjugated system:- Continuous Unhybridized p-orbital parallel to each-other.

- A carbon (or other atom) with an empty p orbital (e.g. a carbocation)
- A carbon with a half-filled p orbital (e.g. a radical)
- A carbon with a lone pair (carbanion)
- Any other atom with a lone pair (e.g. N, O, S, etc.
Types of resonance effect.
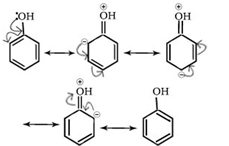
(i)+R effect
Positive resonance or mesomeric effect (+M or +R): The groups show positive mesomeric effect when they release electrons to the rest of the molecule by delocalization. These groups are denoted by +M or +R. Due to this effect, the electron density on rest of the molecular entity is increased.
E.g. -OH, -OR, -SH, -SR, -NH2, -NR2 etc.
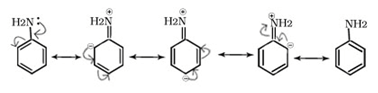
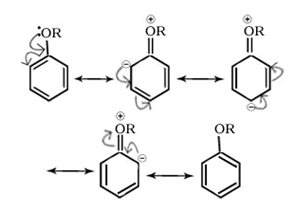
(ii)-R effect
Negative resonance or mesomeric effect (-M or -R): It is shown by substituents or groups that withdraw electrons by delocalization mechanism from rest of the molecule and are denoted by -M or -R. The electron density on rest of the molecular entity is decreased due to this effect.
E.g. -NO2, Carbony group (C=O), -C≡N, -COOH, -SO3H etc.
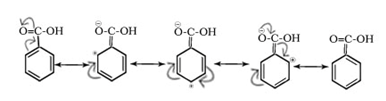
Electromeric effect(E effect)
Transfer of electrons to one of the atom joined by a multiple bond on the demand of an attacking is called electromeric effect.
Types of electromeric effect
(i)+E effect
The transfer of electrons takes place to that atom to which the attacking reagent gets attached

(ii)-E effect
The transfer of electrons takes place to that atom to which the attacking reagent does not get attached.
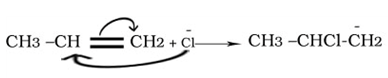
Explain hyperconjugation
The delocalization of sigma electrons of a C-H bond of an alkyl group directly attached to an unsaturated system (or) to a species with an unshared p -orbital such as Carbocations (or) free radicals is known as hyperconjugation. Hyperconjugation is a permanent effect.