Chemistry Notes Some Basic Concept Of Chemistry
Dalton’s atomic theory.
I.Matters are made up of invisible atoms.
II.When atoms of different elements combine with each other they form compound.
III.Atoms of same element have identical properties.
IV.Chemical reaction involve reorganization of atoms .These are neither created nor destroyed in chemical reaction.
What do you mean by precision and accuracy of quantity?
Precision refers to the closeness of various measurements for the same quantity.Accuracy is the agreement of particular value to the true value of results.
Explain the term scientific notation.
Exponential notation in which any number can be represented in the form of N x 10n where n is an
exponent having +ve or –ve value and N is equal to 1-10.Example-scientific notation of 232.508 is 2.32508 X102.
What is significant figure of a number.
Significant figures are meaningful digits which are known with certainty.
Rule 1.All non zeros digits are significant.
Example
325 m has 3 significant figures.
.2584 has 4 significant figures.
Rule 2. Zeros preceding to first non zeros digits are not significant.
Example
.05 m has 1 significant figure.
.0063 has 2 significant figures.
.2584 has 4 significant figures.
Rule 3.Zeros between two non Zeros digits are significant.
Example: 3.008 has 4 significant figures.
Rule 4. Zeros at the end or right of a number are significant provided they are on the right side of decimal point.
Example: .3000 has 4 significant figures.
Rule 5.Terminal Zeros are not significant if there is no decimal point.
Example : 200 has 1 significant figure.
Example 300.0 has 4 significant figures.
Rule 6.Counting number of objects have infinite significant figures.
Example 2 pens have infinite significant figures.
Explain the law of conservation of mass.
According to Lavoisier matter can neither be created nor be destroyed.
Explain the law of definite proportion.
A given compound always contains exactly the same proportion of elements by weight.
Explain the law of multiple proportions.
If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in the ratio of small whole number.
Example: 𝐻2 + 1/2𝑂2 → 𝐻2𝑂
2𝑔 16𝑔 18𝑔
𝐻2 + 𝑂2 → 𝐻2𝑂2
2𝑔 32𝑔 34𝑔
Fixed mass of Hydrogen (2g) combine with the 16 g and 32g of oxygen which is a simple ratio(16:32 or 1:2).
What is Gay Lussac’s law of gaseous volume?
Gases combined or produced in a chemical reaction in simple ratio at same temperature and pressure.
What is Avogadro’s Law?
Equal volume of gases at the same temperature and pressure contain equal no. of molecules.
Define one mole.
One mole is the amount of a substance that contain as many as entities as there are in 12g of carbon
12𝐂 isotope.1 mole of any substance =6.023 x 1023 particles.
What is molar mass?
The mass of one mole of a substance in gram is called its molar mass.
Example=Molar mass of water =18g.
What is mole concept?
(i) When atomic mass is expressed in gram, it gives mass of one mole of that element.
Example: Mass of one mole of sodium atoms =23 gram.
(ii) When molecular mass is expressed into gram it gives mass of one mole of that compound.
Example: Mass of one mole of water (H2O) =18 gram.
(ii) When molecular mass is expressed into u/amu it gives mass of one molecule of that compound.
Example: Mass of one molecule of water (H2O) =18 u.
(iv) When atomic mass is expressed into umu it gives mass of one atom of that element.
Example: Mass of one atom of sodium atom =23 u
(v) Volume of one mole of gas at STP=22.4 liter.
Define amu/u.
One atomic mass unit is defined as a mass exactly equal to one-twelfth of the mass of one carbon atom. 1amu =1.66 x 10-24 g.
What is average atomic mass?
Many naturally occurring elements exist as more than one isotopes. Hence average atomic mass is
calculated.

Define Molecular mass.
Molecular mass is the sum of atomic masses of the elements present in a molecule.
What is formula mass?
Some substance do not exist as a single entity, for these substances instead of molecular mass formula mass is calculated, it denote ratio of two atoms in a given sample of compound.
What do you mean by % composition?
Percentage of particular element in a particular compound is called %composition of that element.

What is empirical formula and molecular formula?
An empirical formula represents the simplest whole number ratio of various atoms present in a compound whereas the molecular formula show the exact number of different atoms present in a molecule.
Explain Stoichiometry concept.
Consider following reaction.
𝐶𝐻4(𝑔) + 2O2(𝑔) → 𝐶O2(𝑔) + 2𝐻2O(𝑔)
The following stoichiometry concepts are.
1.One mole of CH4 reacts with two moles of O2 gas to give one mole of CO2 and two moles of 𝐻2O(𝑔
2. One molecule of CH4 reacts with two molecules of O2 to give one molecule of CO2 and two molecules of H2O.
3. 22.4 of CH4 (g) reacts with 44.8 L of O2 (g)as to give 22.4 L of CO2 (g) and 44.8 L of H2O(g)
4.16g of CH4 reacts with 64g of O2 to give 44g of CO2 and 36 g of H2O.
What is limiting reagent?
The reactant which gets consumed, limits the amount of product formed and is, therefore called the limiting reagent;
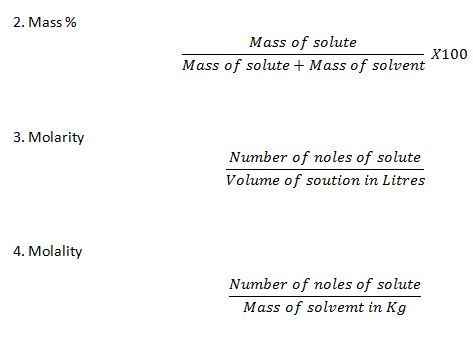
Explain the following concentration terms.
a.Mole fraction
b.Mass %
C.Molarity.
d.Molality.
1.Mole fraction:
The ratio of number of moles of a particular component to the total number of moles of solution is called mole fraction of that component.
let ‘A’ dissolves in substance ‘B’ and their number of moles of 𝑛𝐴 and 𝑛𝐵
respectively, then the mole fraction of A and B are given as-
Mole fraction of A= 𝑛𝐴 ⁄ (𝑛𝐴 + 𝑛𝐵)
Mole fraction of B= 𝑛𝐵 ⁄ (𝑛𝐴 + 𝑛𝐵)